SCUBA DIVING EXPLAINED Questions and Answers on
Lawrence Martin, M.D. Copyright 1997
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Home Brief History of Diving Glossary
|
SECTION HOxygen Therapy For Diving Accidents: At Atmospheric and Hyperbaric PressureWHAT IS OXYGEN THERAPY? Oxygen, of course, is part of the air we breathe. Oxygen is also the most widely prescribed "drug" in hospitals; about a quarter of all patients entering an acute care hospital will receive inhaled oxygen at some point in their stay. Since air already contains 21% oxygen, what doctors prescribe is more accurately known as supplemental oxygen, i.e., an inhaled oxygen concentration greater than the 21% in surrounding air. Sometimes the gas mixture prescribed is called "enriched" air, to distinguish it from "ordinary" air. In hospitals, 100% oxygen is piped into each patient's room, ready for delivery at whatever concentration needed. The actual percentage of oxygen delivered is determined by the type of appliance used to bring the pure oxygen from the wall source to the patient's face, e.g., nasal prongs or various types of face mask. These appliances serve to mix the 100% oxygen from the wall source with the 21% oxygen from ordinary air; depending on the appliance used, the percentage of oxygen delivered to the patient can range between just above 21% to over 90%. The oxygen percentage a doctor orders depends on the clinical condition of the patient. Generally, the lower the patient's oxygen level, the higher the O2 concentration. Pure or undiluted (100%) oxygen is only used rarely, and then only in an intensive care unit. To minimize the risk of oxygen toxicity physicians try to keep the oxygen concentration at 40% or lower. WHAT IS OXYGEN TOXICITY? Oxygen toxicity is damage to some part of the body from inhaling too much oxygen. It is impossible to get oxygen toxicity by inhaling air (21% oxygen) at sea level or lower pressures. A significantly higher percentage of oxygen than the normal 21%, or inhalation at a significantly higher ambient pressure than sea level, can cause oxygen toxicity. As used in hospitals, outside of a hyperbaric chamber, the main risk is lung damage. Under water, inhaling too much oxygen (usually a result of the higher ambient pressures), the main risk is seizures. The specific parameters determining oxygen toxicity - the amount inhaled, the pressure, the duration, etc. - are discussed in Section I. WHAT IS THE DIFFERENCE BETWEEN OXYGEN THERAPY FOR BUBBLE DISEASE AND OTHER MEDICAL CONDITIONS? Supplemental oxygen is widely employed to improve a patient's low oxygen level. Virtually any condition affecting the lungs can lead to a low oxygen level: asthma, bronchitis, emphysema, pneumonia, heart failure, etc. Apart from patients with "bubble disease" - DCS or AGE - if the oxygen level is not reduced there is usually no need to prescribe oxygen therapy. By contrast, oxygen is used in DCS and AGE to shrink bubbles that have formed in the blood and tissues. It is usually not given to improve a low oxygen level. A low blood oxygen level is not the problem in DCS or AGE (unless the lungs are clogged with bubbles, or there is some other direct effect on the lungs, e.g., aspiration from near drowning). In any case, the initial blood oxygen level doesn't matter. For all cases of DCS or AGE the first aid goal is to administer 100% inhaled oxygen. Table 1 summarizes some important differences in oxygen therapy for DCS/AGE (of any cause) and for all other medical conditions. Note that, compared to the universe of patients receiving oxygen, the number treated for DCS or AGE is minuscule. Outside of compressed gas diving, DCS and AGE are rarely encountered, which is one reason why most hospitals don't have a hyperbaric chamber. Scuba diving, however, is very popular, and the role of supplemental oxygen in treating the rare case of DCS or AGE should be understood by all divers. Once extra oxygen is inhaled the gas does not accumulate for later use; it is metabolized right away. The fact that oxygen is not stored by the body is why people die quickly when air is cut off; all available oxygen at that point is completely exhausted in about four minutes. This is also the reason why, if a patient with pneumonia is low on oxygen, it does no good to prescribe "30 minutes of oxygen twice a day." Intermittent dosing may be appropriate for most other drugs, because they stay in the body for some time, but not for supplemental oxygen. To be effective for patients whose blood is low in oxygen, supplemental oxygen has to be supplied and inhaled continuously, until the underlying problem is corrected. (Football players who come off the field for a few whiffs of oxygen on the sidelines are not really benefited in any physiologic sense; by the time they get back in the game the supplemental oxygen they inhaled has been completely used up.)
WHY IS 100% OXYGEN RECOMMENDED FOR ALL VICTIMS OF A SCUBA DIVING ACCIDENT? Scuba accident victims may be low on oxygen, especially if they have aspirated water or developed some other lung complication, but the main reason for 100% oxygen therapy is to shrink any gas bubbles formed during ascent. Treatment of gas bubbles requires 100% oxygen regardless of the blood oxygen level, and for as long as is practical; just how long is governed by the risk of oxygen toxicity. (Note. Examination of the patient is not a reliable guide to the blood oxygen level. A patient can be in distress with a normal oxygen level, or appear calm and peaceful while succumbing from hypoxia. Neither respiratory rate and nor the skin color are reliable guides to hypoxia. Hospitals can measure the patient's oxygen level in various ways. It is unlikely that any test for blood oxygen will be available at the site of a diving accident but, for reasons stated, none is really needed.) Unlike the typical patient with a low oxygen level (from pneumonia, heart failure, asthma, etc.), the victim of bubbles from diving should benefit from even a short period of 100% oxygen. One hundred percent oxygen may prevent existing bubbles from expanding, or even shrink them enough to provide symptomatic relief. The main reason to have supplemental oxygen available at the site of any scuba dive is to treat DCS or AGE, two of the most serious dive-related problems. For either problem hyperbaric oxygen therapy is optimal, but until transport can be arranged to a hyperbaric chamber, the victim should receive 100% oxygen at atmospheric pressure, via a face mask. The secondary reason to have supplemental oxygen available is to treat hypoxia (low oxygen level), such as is found in victims of near-drowning accidents. Oxygen can be very helpful for any hypoxic victim, but that is not the primary reason for having oxygen available when diving. Some might argue that this is quibbling over reasons, but nonetheless there are two very different indications for supplemental oxygen in dive accidents. It is primarily as treatment for DCS/AGE that oxygen is recommended at every dive site. REASONS TO HAVE OXYGEN AT EVERY DIVE SITE Primary: To treat DCS/AGE Secondary: To treat hypoxia (e.g., near-drowning, shock) No matter what the indications, when oxygen is used as part of first aid for a diving victim it is always given at atmospheric pressure (i.e., not under hyperbaric conditions). HOW IS OXYGEN USED AT ATMOSPHERIC PRESSURE? On land, outside of a hyperbaric chamber, oxygen is always delivered at the ambient (atmospheric) pressure. The oxygen concentration administered can vary from just over 21% up to the maximum, 100%. At sea level the ambient pressure is 760 mm Hg, also known as "one atmosphere" (1 atm.; 14.7 psi). At an altitude of 18,000 feet the ambient pressure is half that at sea level, and so equals 0.5 atm (7.35 psi). It should be apparent that the most oxygen a patient can receive on land, outside of a hyperbaric chamber, is 100% given at sea level pressure; this is one atmosphere of oxygen and is abbreviated "1 atm. O2". (You could get a slightly higher oxygen pressure if you administer 100% oxygen on land below sea level. The few habitable areas below sea level are so close to sea level that their ambient pressure can be considered 1 atm.) In Denver, where the average atmospheric pressure is about 85% of sea level, 100% inspired oxygen equals .85 atmosphere of oxygen (.85 atm O2). One hundred per cent oxygen delivered at 18,000 feet, where the ambient pressure is 0.5 atmosphere, is 0.5 atm. O2, etc. As pointed out in Section D, it is important to keep the different pressure terms clear. There are several terms in common use that mean the same thing; the box below reviews some definitions discussed earlier. when surrounded by air: ambient pressure = atmospheric pressure = barometric pressure 1 atmosphere = the average air pressure at sea level = 760 mm Hg = 14.7 psi; 0.5 atmosphere = � the average air pressure at sea level = 380 mm Hg = 7.35 psi; etc. when surrounded by water: ambient pressure = water pressure + atmospheric pressure above the water = atmospheres of air pressure at that depth (e.g., a depth of 66 fsw is 3 atmospheres, 99 fsw is 4 atmospheres, etc.) WHAT ARE ATMOSPHERES OF OXYGEN? Atmospheres of oxygen are based on the O2 concentration in the inspired gas mixture and the ambient pressure. One atm. O2 is 100% oxygen delivered at one atmosphere of pressure; other combinations of concentration and pressure can also equal one atm. O2. Study the following relationships for one, two and three atm. O2 to solidify the concept of atm. O2 (fsw = feet sea water). Then study Figure 1, which shows the rise in atmospheres of oxygen with depth, while breathing compressed air.
TEST YOUR UNDERSTANDING Answers Figure 1. Atmospheres of O2 (vertical axis) while breathing compressed air at various depths (horizontal axis). (fsw = feet sea water; sl = sea level)
HOW IS OXYGEN USED AT THE DIVE SITE? Standard resuscitation and treatment protocols, including CPR when necessary, should be followed in any life-threatening situation. The information provided herein is intended only as a general discussion of the rationale and use of oxygen therapy for suspected bubble disease. Medical and first aid texts, several of which are listed in the bibliography, can be consulted for more detailed information on how to provide oxygen, transport the patient, etc. Oxygen toxicity should not be a concern when giving oxygen as part of first aid. If possible, always give 100% oxygen via a positive pressure (tight fitting) mask; this concentration of O2 is part of first aid for any scuba diving accident. At the same time, arrange to transport the victim to an appropriate medical facility. Do not worry about oxygen toxicity unless 100% oxygen is administered continuously for more than two hours. At that point, if an appropriate medical facility has not been reached and supplemental oxygen is still available, an air break of 15-30 minutes should begin, followed by continuation of 100% oxygen. If the diver's symptoms abate during treatment, 100% O2 is still recommended if either DCS or AGE is suspected; bubbles could reform and symptoms could recur. However, don't insist on a tight fitting mask if the diver finds it very uncomfortable or is vomiting; you can alternate with a loose fitting oxygen mask. In first aid for a diving accident, it is always more important to give some oxygen than none at all. Since 1991 DAN has sponsored half-day courses on oxygen therapy for certified divers. The DAN Oxygen First Aid in Dive Accidents Course, usually held at a local dive shop, uses both lecture and hands on experience to teach three important aspects of first-aid oxygen therapy: 1) how to make oxygen available at any dive site; 2) how to use the recommended equipment; 3) the advantages of oxygen therapy for dive accident victims. 1) OXYGEN AVAILABILITY. For field use oxygen comes in small green tanks (typical capacity about 40 cu. ft. at 1500 psi) that look like small scuba tanks, but contain 100% oxygen instead of compressed air (Figure 2). Tanks of oxygen are always painted green, either the entire tank (if made of steel) or the top portion (if aluminum). DAN recommends, and good practice mandates, that all dive boats and dive facilities carry a readily accessible tank of oxygen, an oxygen regulator and appropriate face masks for first aid use. In fact, it is recommended that one should avoid diving from a boat that does not have oxygen on board. Each year more and more dive boats are carrying oxygen.
Figure 2. DAN oxygen tank and equipment for field use. (Courtesy Diver's Alert Network) 2) HOW TO USE. A principal focus of the DAN Oxygen Course is on how to use the equipment. It does no good for the distressed diver if the boat contains a tank of oxygen that no one knows how to use. (Note: This section is for informational purposes only. Formal training in both CPR and use of oxygen equipment should be obtained from qualified instructors in a hands-on situation.) As with any tank of compressed air, a regulator is required to lower the pressure so it can be safely delivered to the diver. Scuba air tank regulators cannot be used for this purpose. You need a special O2-tank regulator. A green plastic tube delivers oxygen from the regulator to the mask, which fits over the diver's mouth and nose. (Advantages of oxygen therapy are discussed later in this chapter.) WHAT ARE THE TYPES OF OXYGEN MASKS AVAILABLE FOR DIVE ACCIDENT VICTIMS? Three types of oxygen delivery masks are available in the DAN oxygen kit: a demand-valve-with-mask, a pocket mask, and a non-rebreathing mask (Table 2, Figures 3 and 4). The first two masks can deliver close to 100% oxygen to the diver; the last one can deliver about 70-80% oxygen. The demand-valve-with-mask is similar to the demand valve found in all second stage scuba regulators; with each inhalation, it delivers compressed gas (100% oxygen) to the diver's mouth and nose under a slight positive pressure. As with a second stage regulator, the diver must first initiate a breath for the gas to be delivered; thus the demand-valve-with-mask cannot be used on the non-breathing victim. To assure close to 100% oxygen, the mask must make a tight seal with the diver's face (i.e., no air leaks around the mask). The positive pressure given by the demand-valve-with-mask is only slightly higher than atmospheric. If atmospheric pressure is 760 mm Hg, the pressure inside this mask on inhalation may be 770 mm Hg, or only about .01% higher. This is obviously not hyperbaric therapy, which is two or more times atmospheric pressure (i.e., 1520 mm Hg or greater). The extra pressure inside the mask helps facilitate entry of pure oxygen into the diver's lungs.
For the victim who is not breathing (apneic), the pocket mask can be used; it is so-called because the mask easily folds and fits into one's pocket (Figure 4). The pocket mask is designed to fit snugly over the victim's mouth and nose. As long as the mask-face seal is airtight, the victim can be ventilated through a port on the mask by the rescuer using artificial respiration but without mouth-to-mouth contact The pocket mask technique of resuscitation is a variation of standard mouth-to-mouth breathing taught in basic CPR. (Obviously, if the victim is also pulseless cardiac compressions must be delivered at the same time.) A plastic tube connects the oxygen regulator to the mask, so that each breath delivered by the rescuer should provide a high concentration of oxygen. A one-way valve diverts the victim's exhaled air away from the rescuer.
Figure 3. DAN's 'mini' charter boat oxygen unit, showing oxygen regulators (lower right), demand valve with mask, lower left, and non-rebreather face mask (attached to box cover); oxygen tank is not shown. (Courtesy Diver's Alert Network) The third type of mask is called a non-rebreather (Figure 3) because it does not allow the victim to rebreathe any of his own exhaled air; the flow of oxygen-enriched air through the mask is great enough to quickly wash away exhaled air. It is an excellent backup mask to the first two types, and can deliver somewhere around 70-80% oxygen. The non-rebreather is useful for the victim who can't tolerate the positive pressure mask, or who is vomiting. Also, if there are two dive accident victims, the non-rebreather can be used for one while the demand-valve-with-mask is used for the other (both masks are fed from a single tank of oxygen and single regulator). One disadvantage of the non-rebreather is that it delivers oxygen continuously instead of only on demand, and so is relatively wasteful of the gas. DAN markets all the necessary oxygen therapy equipment (regulator, masks) in one convenient kit, available to oxygen course instructors and dive shop/boat operators. DAN will also supply the oxygen tank and a training mannequin, as well as a special carrying case for boats (see Section R for DAN's address).
Figure 4. Pocket mask for oxygen administration. (Courtesy Diver's Alert Network.) WHAT ARE THE ADVANTAGES OF SUPPLEMENTAL OXYGEN THERAPY FOR DIVE ACCIDENT VICTIMS? The overall goals of supplemental oxygen therapy are to hasten recovery, preserve organ function, save a life. Behind these goals are two basic reasons for using 100% oxygen when bubble disease (AGE or DCS) is suspected: If the victim is hypoxic (low in oxygen), the extra oxygen might raise the blood oxygen level enough to provide more (and sufficient) oxygen for the brain, heart and other vital organs. The extra oxygen will help shrink existing nitrogen or air bubbles and prevent others from forming, and so provide important first aid while the victim is transported to a hyperbaric chamber. The victim may experience symptomatic relief with just 100% oxygen delivered at atmospheric pressure. WHAT IS THE PHYSIOLOGIC BASIS FOR OXYGEN THERAPY? The two stated reasons for oxygen therapy can be better understood by reference to blood oxygen pressures. Oxygen pressure in the blood is called the PaO2: partial pressure (P) of oxygen (O2) in the arterial blood (a). At sea level and breathing air (21% O2), normal PaO2 is about 80 to 100 mm Hg. This means that the oxygen pressure in the blood, by itself, will support a column of mercury 80 to 100 mm high. Because air pressure falls with altitude, the normal PaO2 also falls with altitude. In Denver, for example, the normal PaO2 is only 65 to 85 mm Hg. On the summit of Mt. Everest PaO2 has been estimated in air-breathing climbers to be only about 28 mm Hg! (Any lower and the climbers would not have lived to report the ascent.) If a healthy person inhales 100% oxygen at sea level, nitrogen in the lungs and tissues is replaced by the pure oxygen. This "washout" of nitrogen by 100% oxygen is reflected in a much higher PaO2, about 600 mm Hg at sea level. (During the washout with 100% O2 at sea level, the blood nitrogen pressure actually falls from about 573 mm Hg to almost zero.) While PaO2 goes up markedly with 100% oxygen, the actual number of oxygen molecules in the blood goes up only slightly. A PaO2 of 600 mm Hg puts only about 7% more oxygen molecules into the blood than a PaO2 of 100 mm Hg, even though the oxygen pressure is six times higher. WHY DOES A PaO2 SIX TIMES NORMAL PROVIDE ONLY 7% MORE OXYGEN IN THE BLOOD? The point made in the last paragraph may be confusing and it bears some elaboration. The reason a given percentage increase in PaO2 does not translate into the same percentage increase in oxygen molecules (oxygen content) is because the blood hemoglobin is almost fully saturated with oxygen at a PaO2 of 100 mm Hg. In the normal situation, 98% of all oxygen in the blood is carried by hemoglobin; the other 2% is dissolved in the plasma. Oxygen pressures above 100 mm Hg add only dissolved oxygen to the blood; at an ambient pressure of one atmosphere (sea level), this extra dissolved oxygen is a small amount when compared to the hemoglobin-bound oxygen, and not enough to make a big difference to patients with lung or heart disease. Table 3 shows a range of PaO2 values that can be achieved by varying the O2 concentration inhaled at one atmosphere. Note that oxygen content in-creases significantly until PaO2 is in the normal range; beyond 100 mm Hg, only about 0.3 volumes % of oxygen are added for every 100 mm Hg increase in PaO2. When the PaO2 is six times normal the blood contains just 7.5% more oxygen molecules. Thus there is a major distinction between oxygen pressure and oxygen content in the blood. Oxygen pressure directly reflects the pressure of inhaled oxygen, and is due solely to the unbound (dissolved) fraction of oxygen in the blood; it is the same value regardless of the hemoglobin content. Oxygen content, on the other hand, reflects the actual number of oxygen molecules in the blood, both bound to hemoglobin and unbound (dissolved). A low hemoglobin value will not affect a diver's oxygen pressure, but will have a direct effect in reducing oxygen content. Table 3 shows these differences.
Inhaling 100% oxygen at one atmosphere of pressure (sea level) adds greatly to oxygen pressure, but very little to the oxygen content. That is why, when the goal is to improve a low PaO2 (the situation in virtually all non-diving-accident patients who receive oxygen therapy), there is no reason to exceed the normal PaO2 range of 80-100 mm Hg, especially as it may add some risk of oxygen toxicity. The goal is different for victims of AGE or DCS, where the principal rationale for oxygen therapy is to shrink gas bubbles in the tissues and circulation. The higher the oxygen pressure in the blood, the faster nitrogen is kicked out of the bubbles and the quicker they will shrink. The limiting factor in maintaining high oxygen pressures in victims of DCS/AGE is oxygen toxicity.
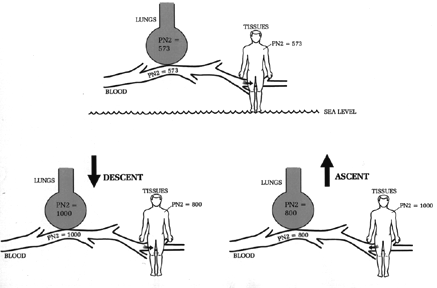
TEST YOUR UNDERSTANDING Answers WHY DOES REPLACING NITROGEN WITH OXYGEN SHRINKBUBBLES? Nitrogen and oxygen are handled very differently in the body. Nitrogen is inert; it is not metabolized by the tissues. It is just there. Under ordinary (non-diving) conditions, the pressure of nitrogen in our blood and tissues is the same as in our lungs and in the atmosphere (Figure 5). Diving upsets this equilibrium because it subjects you to changing ambient pressures. As you descend breathing compressed air, the pressure of inhaled air (and its individual components) changes with surrounding water pressure. The pressure of inhaled nitrogen and oxygen increase, and so more nitrogen and oxygen molecules enter the blood and tissues (according to Henry's Law). Figure 5 also shows that on descent there is a nitrogen pressure gradient from inhaled air and lungs (highest) to blood (intermediate) to tissues (lowest). Nitrogen, being inert, accumulates in the body with every dive. Oxygen, by contrast, is metabolized and doesn't accumulate in the tissues to any great extent. (Oxygen toxicity comes from high inspired oxygen pressures, not from any accumulation of oxygen molecules in the body). The extra nitrogen that accumulates on descent begins to leave on ascent (Figure 5). As the ambient pressure decreases the nitrogen pressure gradient reverses, so that nitrogen pressure is highest in the tissues, intermediate in the blood and lowest in the lungs. Without this reverse gradient divers would never remove the excess nitrogen accumulated on any dive. DCS occurs when the nitrogen that accumulated in the tissues on descent comes out too fast on ascent; instead of dissolving harmlessly in the blood, from where it can be exhaled, it forms nitrogen bubbles large enough to inflict pain or cause blockage of blood flow.
Figure 5. Relative pressures of nitrogen in air and blood. When not diving there is no gradient (top). Nitrogen pressure increases with each dive. Because it takes time for nitrogen to equilibrate, there is a gradient from lungs to tissues with descent (lower left), and from tissues to lungs with ascent (lower right). The origin and composition of bubbles differ between AGE and DCS, but the principle of treatment is the same for both: exchanging nitrogen in the bubbles for oxygen. Both types of gas bubbles contain a high nitrogen content. In AGE bubbles it is 78% (same as ordinary air), and in DCS bubbles it is near 100%. Since nitrogen concentration in AGE bubbles is the same as in the blood and air (78%), there is no gradient for nitrogen to leave the bubbles. Eventually some oxygen from inspired air finds its way into the bubbles and they do shrink, but that is a slow process, "too little, too late" for the victim. Treating AGE (and DCS) requires hastening shrinkage of bubbles, and there are only two ways to accomplish this: compress the bubbles by increasing the ambient pressure, or speed up nitrogen's exit from the bubbles. Increasing the ambient pressure requires a hyperbaric chamber. (In theory, sending the diver back down in the water would also work, but this is a tricky form of therapy and impossibly dangerous for any seriously ill diver. Although in-water recompression is a routine practice in some remote commercial dive operations, it is never recommended for the recreational diver. Without a full face mask for the diver, and professional support personnel both below the water and on the surface, sending an ill recreational diver back down is an invitation for disaster.) In lieu of a hyperbaric chamber, supplemental oxygen is used to hasten nitrogen's exit from the bubbles. If effect, 100% inspired oxygen is used to de-nitrogenate the blood and shrink any gas bubbles. As the victim inhales 100% oxygen, nitrogen in the blood is "kicked out" by the oxygen and is exhaled by the lungs. Initially, the bubbles still contain a high nitrogen pressure. However, as nitrogen leaves the blood (replaced by oxygen), a large nitrogen gradient forms between the inside of the bubble and the surrounding blood; this fosters nitrogen's exit from the bubble (Figure 6). At the same time that nitrogen exits, some oxygen enters the bubble. However, because this O2 is given up to the tissues for metabolism, there remains a net loss of gas molecules from the bubble and the bubble shrinks. In summary, the purpose in giving 100% O2 to anyone with suspected bubble disease is to rid the blood of nitrogen, so that a large nitrogen gradient forms between inside and outside the bubble. In this manner nitrogen flows out of the bubbles and into the blood, from where it can be excreted by the lungs. This principle of therapy requires only a high inspired oxygen concentration; it does not require a hyperbaric chamber. Pure oxygen is the preferred washout gas because it contains no nitrogen, and the extra oxygen does not accumulate in the body. The risk of oxygen toxicity can be reduced by decreasing the concentration of inhaled oxygen (e.g., a mixture of 50% O2 + 50% N2), but this reduces the gradient for nitrogen diffusion nitrogen egress.
Figure 6. Nitrogen in bubbles with supplemental oxygen. Fortunately, it is not difficult to get oxygen into the bubbles. Under normal circumstances there is always a positive pressure gradient for oxygen between air sacs in the lungs and the blood bathing them; otherwise, no oxygen would enter the blood and be delivered to the tissues. Likewise, there is a positive pressure gradient between oxygen in the blood and inside the bubbles; and a positive gradient between oxygen in the bubbles and the tissues. Thus, when supplemental O2 is given, oxygen will flow:
A bubble containing air will shrink slowly, as it contains 78% non-metabolized nitrogen (which moves nowhere) and only 21% oxygen. A bubble containing pure nitrogen (as found in DCS) will shrink even more slowly; that it shrinks at all is due to the fact that some oxygen eventually finds its way into the bubble, replacing nitrogen. HOW IS OXYGEN THERAPY USED AT HYPERBARIC PRESSURES? Unless trained in hyperbaric medicine, you will never be called upon to administer this type of therapy. Even so, all divers should be aware of the role of hyperbaric chambers in treating bubble disease. Hyperbaric chambers are heavy, rigid structures that can hold one or more people in an environment of high ambient pressure. Every hyperbaric chamber treatment is like a compressed air dive, only there is no water and the limits of compression are rigorously controlled by the hyperbaric operator. In the U.S. there are about 300 operating hyperbaric chambers; about 68% of them are the monoplace type (Figure 7). Monoplace means that only one person can fit inside the chamber at a time. Multiplace chambers can accommodate two or more people, depending on their size. In addition to treating more than one patient at a time, multiplace chambers offer a great deal more flexibility than monoplace chambers: A hyperbaric tender (nurse or other medical assistant) can accompany the patient inside the multiplace chamber and administer to his or her needs. Tenders can rotate in and out of the chamber while the patient continues to receive treatment. Any ancillary equipment needed can be easily entered into the chamber, including a mechanical ventilator. The patient can receive 100% oxygen through a head tent or face mask, while the tender is breathing compressed air.
Figure 7. Monoplace hyperbaric chambers. Courtesy of Perry Baromedical. Hyperbaric therapy can be administered with any percentage of oxygen from 21% (ordinary air) to 100%. Either way, hyperbaric therapy will increase the oxygen pressure in the patient's blood. (Two atm. O2 can be achieved at 33 fsw breathing 100% O2, or at 282 fsw breathing compressed air). Two fundamental principles of hyperbaric therapy hold regardless of the type of chamber used, the concentration of oxygen inhaled, the ambient pressure achieved, or the duration of therapy: 1) The higher the ambient pressure, the greater the shrinkage of bubbles. Whereas 100% oxygen at atmospheric pressure can only denitrogenate the blood and hasten nitrogen out of the bubbles, hyperbaric pressures will actually compress the bubbles. The higher ambient pressure inside the chamber increases the gas pressure in the blood, which in turn compresses the bubble according to Boyle's law. Even with minimal compression the victim may find immediate pain relief. When the hyperbaric pressure is removed (equivalent to an ascent) the bubble could re-expand; however, it does not re-expand to the same pre-compression size, because its nitrogen content is also reduced during compression. This is where oxygen plays a vital role in hyperbaric therapy. 2) The higher the blood oxygen pressure, the faster the gas bubbles will shrink. Hyperbaric oxygen therapy greatly increases oxygen pressure in the blood, driving oxygen into the bubbles and hastening nitrogen's exit. This is the same mechanism as when 100% O2 is given under atmospheric pressure, but greatly accelerated. Thus there are two reasons for bubble shrinkage from 100% O2 inhaled under pressure:
If oxygen was not metabolized, merely replacing nitrogen with oxygen would not decrease the bubble's size; however oxygen, once inside the bubble, then enters the tissues, where it is metabolized. Thus the bubble shrinks. Hyperbaric oxygen therapy (HBO) goes one giant step further than is possible with 100% oxygen at atmospheric pressure. Whereas one atm. O2 is achievable without a hyperbaric chamber, with a chamber the patient can receive two, three or any number of O2 atmospheres. Because hyperbaric therapy with air actually puts more nitrogen into the patient's blood (albeit while shrinking the bubbles), most hyperbaric physicians prefer using short periods of 100% hyperbaric oxygen in treating bubble disease rather than just hyperbaric air. Table 4 shows expected changes in the blood of subjects undergoing hyperbaric therapy with 100% oxygen (data in this table assumes the patient has normal lungs). Although patients may respond differently at each level of therapy, keep in mind the principles stated above.
Diving accidents are just one of several conditions handled by most hospital hyperbaric centers. There is much controversy about hyperbaric therapy in some diseases. The Undersea and Hyperbaric Medical Society (10531 Metropolitan Ave., Kensington, MD 20895) has developed a list of approved conditions that are generally reimbursable by third party payers. This list includes:
For non-diving conditions, hyperbaric therapy aims to raise blood oxygen pressure much higher than achievable by 100% O2 at one atmosphere in order to put more oxygen into the blood. For DCS and AGE, on the other hand, the goal is to replace any nitrogen in the gas bubbles with oxygen, so the bubbles will shrink faster. Note that with HBO therapy the extra dissolved oxygen in the blood can appreciably increase the blood's total oxygen content (Table 4). One hundred percent oxygen at three atmospheres can add an extra 5 vol.% to the blood, about a quarter of the normal value. In extremely anemic patients this extra dissolved oxygen can be important therapeutically, and in some cases sustain a life that might otherwise be lost because of low oxygen content. In bubble disease, however, HBO is given primarily to shrink the bubbles, not to increase the dissolved oxygen content.
TEST YOUR UNDERSTANDING Answers WHAT IS A HYPERBARIC TREATMENT TABLE? The U.S. Navy has developed treatment tables for DCS and AGE. An example of the DCS table was shown in Figure 2, Section G. Note that these tables are always amenable to modification by the physician treating the patient. However, all treatment tables alternate a high concentration of oxygen (usually 100% ) with periods of breathing air. The reason, of course, is to prevent oxygen toxicity, a potential hazard of hyperbaric oxygen therapy. The specific treatment table chosen, and the number of times it is administered, will depend on the clinical course of the patient and assessment of the hyperbaric physician. Following is a case history. About 30 minutes after J.W. surfaced from his second dive of the morning he felt numbness and tingling in his legs. This dive, 60 feet for 40 minutes, followed a first dive to 110 feet for 10 minutes. However, he admitted to diving "for just a few seconds" to 120 feet on his first dive, to chase a large grouper. On the boat J.W. received 100% O2 by positive pressure face mask. The boat captain radioed ahead for an ambulance to meet at the dock. When the boat docked 30 minutes later, J.W. could not walk; he had near total paralysis of both legs but was mentally alert. He was carried off the boat and into the waiting ambulance. The closest hyperbaric facility was one hour away, in a local hospital. The ambulance attendant called ahead to have the chamber ready. J.W. continued to receive 100% oxygen en route. In the emergency room he was quickly examined by the hyperbaric physician on duty. Diagnosis: Type II DCS, bubble damage to the spinal cord. A catheter was inserted into J.W.'s bladder so he could urinate, and an intravenous line was started for fluids. Approximately 15 minutes after arrival to the hospital J.W. entered the multiplace chamber with a nurse tender; treatment was begun on U.S. Navy Table 6. At the end of treatment he felt considerably better and could move his legs a little, but still felt some numbness. He stayed in the hospital and over the next week received five more hyperbaric treatments. By the end of the week he could walk unassisted. He was discharged at that time, with only some residual decrease in sensation, which improved over time. J.W. believes he became "bent" solely because he exceeded the diving tables. He plans to dive again and pay much more attention to his depth and bottom time. His physician cautioned him to be very conservative and to dive well within the dive tables. J.W. was fortunate. The dive boat carried oxygen, a chamber was in the area, he never developed cerebral symptoms (mental confusion, stroke, etc.), and his paralysis cleared with hyperbaric therapy. Answers to TEST YOUR UNDERSTANDING1. a. 3 atm. b. 63 atm. O2 (three times sea level PO2) c. 2.34 atm. N2 (three times sea level PN2) 2. a. 5 atm.; 7.35 psi b. 105 or .11 atm. O2 (sea level is .21 atm. O2) c. 5 atm. O2 3. a. 1.33 atm. O2 b. he is at the threshold of risking oxygen toxicity c. 33 fsw 4. d. The maximum oxygen concentration is 100%. The other answers represent one atm. of inhaled oxygen. 5. d. Only oxygen content correlates with the number of oxygen molecules in the blood. 6. true 7. false 8. false 9. false 10. true 11. true REFERENCES AND BIBLIOGRAPHY Quoted sources and general references are listed by section or sections, in alphabetical order. An asterisk indicates references that are especially recommended. Medical textbooks and journal articles can be obtained from most public libraries via inter-library loan. SECTION H. Oxygen Therapy: At Atmospheric and Hyperbaric Pressure See References for Sections b-e, plus the following: DAN Oxygen Provider Course Manual. Divers Alert Network, Durham, NC. Updated frequently. DAN Underwater Diving Accident Manual including Oxygen First Aid Manual. Divers Alert Network, Durham, NC. Updated frequently. Donaldson, K. Oxygen and the Diver. Hendrick W, Thompson B. Oxygen and The Scuba Diver. Best Publishing Co., Flagstaff, AZ, 1993. Lippmann J. Oxygen First Aid for Divers. Divers Alert Network, 1993. Martin L. Pulmonary Physiology in Clinical Practice. C.V. Mosby Co., St. Louis, 1987. Wright SE, Jenkinson SG. Clinical use of hyperbaric oxygen. Clinical Pulmonary Medicine
1994;1:237-249. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||