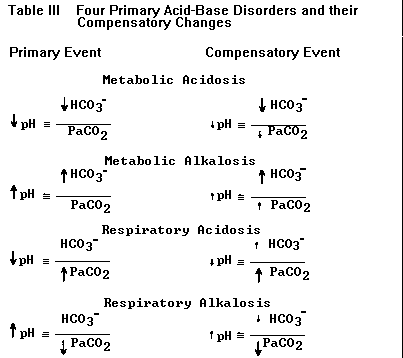
c) By convention 'acidosis' and 'alkalosis'
refer to in-vivo physiologic derangements and not
to any change in pH. Each primary acid-base
disorder arises from one or more specific clinical
conditions, e.g., metabolic acidosis from diabetic
ketoacidosis or hypoperfusion lactic acidosis;
metabolic alkalosis from diuretics or nasogastric
suctioning; etc. Thus the diagnosis of any
primary acid-base disorder is analogous to
diagnoses like "anemia" or "fever"; a specific
cause must be sought in order to provide proper
treatment. Because of the presence of more than
one acid-base disorder ('mixed disorders') a
patient with any acidosis or alkalosis may end up
with a high, low or normal pH. For example, a
patient with obvious metabolic acidosis from
uremia could present with a high pH due to a
concomitant metabolic alkalosis (which may not be
as clinically obvious). Acidemia (low pH) and
alkalemia (high pH) are terms reserved for
derangements in blood pH only.
d) Compensation for a primary disorder takes
place when the other component in the H-H ratio
changes as a result of the primary event; these
compensatory changes are not classified by the
terms used for the four primary acid-base
disturbances.9-10 For example, a patient who
hyperventilates (lowers PaCO2) solely as
compensation for metabolic acidosis does not have
a primary respiratory alkalosis but simply
compensatory hyperventilation. This terminology
helps separate diagnosable and treatable clinical
disorders from derangements in acid-base that
exist only because of the primary disorder.
e) Compensatory changes for acute respiratory
acidosis 11 and alkalosis,12 and metabolic
acidosis 13,14 and alkalosis,15,16 occur in a
predictable fashion, making it relatively easy to
spot the presence of a mixed disorder in many
situations. For example, single acid-base
disorders do not lead to normal pH. Two or more
disorders can be manifested by normal pH when they
are opposing, e.g., respiratory alkalosis and
metabolic acidosis in a septic patient. Although
pH can end up in the normal range (7.35-7.45) in
single disorders of a mild degree when fully
compensated, a truly normal pH with abnormal HCO3-
and PaCO2 should make one think of two or more
primary acid-base disorders. Similarly, a high pH
in a case of acidosis or a low pH in a case of
alkalosis signifies two or more primary disorders.
f) Maximal respiratory compensation for a
metabolic disorder takes about 12-24 hours and
maximal renal compensation for a respiratory
disorder takes up to several days. As a rule of
thumb, in maximally compensated metabolic acidosis
the last two digits of the pH approximate the
PaCO2.17 For example, a patient with a disease
causing uncomplicated metabolic acidosis over 24
hours' duration, whose pH is 7.25, should have a
PaCO2 equal or close to 25 mm Hg. In metabolic
alkalosis respiratory compensation is more
variable and there is no simple relationship by
which to predict the final PaCO2.16
CASE 2. A 31-year-old woman presented to the
emergency room with mild diabetic
ketoacidosis (DKA) and dyspnea; arterial pH
was 7.25, PaCO2 34 mm Hg, HCO3- 16 mEq/L,
blood sugar 475 mgm%. Her breathing
difficulty was attributed to Kussmaul-type
respirations characteristic of DKA. Judging
her DKA non-critical, the admitting physician
placed her on a general medical ward and
began appropriate treatment with insulin and
fluids. Four hours later she appeared more
dyspneic; repeat blood gas showed pH 7.18,
PaCO2 49 mm Hg, HCO3- 18, blood sugar 350
mgm%. She was transferred to MICU where she
was noted to be wheezing; bronchodilator
therapy was begun. Her pre-bronchodilator
peak expiratory flow rate was 110 L/min, 25%
of predicted. Two days later her
ketoacidosis was fully corrected and peak
flow was recorded at 350 L/min.
The mistake here was in not appreciating the
patient's lack of appropriate hyperventilation for
a state of ketoacidosis, and therefore in not
diagnosing her respiratory impairment (she was not
wheezing on arrival to ER). Similar cases have
been reported in the literature.18
g) Acute, uncompensated respiratory alkalosis
(acute hyperventilation) and acidosis (acute
hypoventilation) cause predictable changes in pH
and bicarbonate11,12 (Table IV). Bicarbonate
increases slightly from the biochemical reaction
of acutely retained CO2 and decreases when CO2 is
acutely excreted;11,12 these changes are
instantaneous and independent of any renal
compensation. Extreme acute hyperventilation can
lower the bicarbonate to about 15 mEq/L and
extreme acute hypoventilation can raise it to
about 29 mEq/L (Table IV); a bicarbonate value
outside this range must indicate either a renal
compensatory mechanism or a primary metabolic
acid-base disorder. The biochemical changes in
bicarbonate from acute shifts in PaCO2 point to
another particularly useful clue to the presence
of a mixed disorder: a higher- or lower-than-
expected bicarbonate value with any change in
PaCO2. Thus a slightly low HCO3- concentration in
the presence of hypercapnia suggests a concomitant
metabolic acidosis (e.g., PCO2 50 mm Hg, pH 7.27,
HCO3- 22 mEq/L); a slightly elevated
HCO3-
in the presence of hypocapnia suggests a concomitant
metabolic alkalosis (e.g. PCO2 30 mm Hg, pH 7.56,
HCO3- 26 mEq/L).
TABLE IV. Changes in arterial pH and bicarbonate with acute
changes in PaCO2. The ranges represent the 95% confidence limits
for pH and bicarbonate when PaCO2 changes acutely (before any
renal compensation takes place). Note that bicarbonate decreases
with acute hyperventilation and increases with acute
hypoventilation. (Data from references 11-12).
TABLE IV
| PaCO2 (mm Hg) |
pH |
HCO3- |
| 15 |
7.61-7.74 |
15.3-20.5 |
| 20 |
7.55-7.66 |
17.7-22.8 |
| 30 |
7.45-7.53 |
21.0-25.6 |
| 40 |
7.38-7.45 |
22.8-26.8 |
| 50 |
7.31-7.36 |
24.1-27.5 |
| 60 |
7.24-7.29 |
25.1-27.9 |
| 70 |
7.19-7.23 |
25.7-28.5 |
| 80 |
7.14-7.18 |
26.2-28.9 |
| 90 |
7.13-7.09 |
26.5-29.2 |
h) The bicarbonate (or total CO2) should also be examined in
relation to the other measured electrolytes, specifically to
calculate the anion gap (AG). AG is the Na+ concentration minus
(total CO2 + Cl-). The normal AG, 12 +/- 4 mEq/L, is an
artifact of measurement since these three electrolytes are only the
ones most commonly measured. (Since the value of K+ is small
and relatively constant it is not usually used to calculate the AG;
if K+ is used then the normal AG is about 16 +/- 4 mEq/L). If all
the serum anions and cations were measured anions would equal
cations and there would be no anion gap. The importance of the
anion gap is that it can help both to diagnose the presence of a
metabolic acidosis and characterize its cause. Thus, regardless of
pH an elevated AG suggests a metabolic acidosis from unmeasured
organic anions, e.g., lactic acidosis or ketoacidosis;19-21 the higher
the AG the more likely it reflects an organic acidosis.19 On the
other hand a normal AG in a patient with metabolic acidosis
indicates a hyperchloremic acidosis, most commonly from renal or
gastrointestinal bicarbonate loss, e.g., renal tubular acidosis or
diarrhea.
* * *