Diagnosing Mixed Acid-Base Disorders
The following section is adapted from Chapter 8 of
All You Really Need to Know to Interpret Arterial Blood
Gases, 2nd edition.
Does the patient have a mixed acid-base disorder?
There is perhaps no more confusing topic related to blood gases than mixed acid-base disorders.
This topic has already been discussed in Chapter 7 in regards to the serum electrolytes. As was pointed
out, calculation of the anion and bicarbonate gaps can help uncover mixed metabolic disorders (e.g.,
anion gap metabolic acidosis and metabolic alkalosis). Of course, to accurately diagnose the specific
disorders and their relative severity at least one set of blood gases is usually necessary.
When there is significant deviation from the expected changes for a single disorder, a mixed
disorder is usually present. I have found the following four "tips" especially helpful in diagnosing
mixed acid-base disorders.
|
TIP 1. Don't interpret any blood gas data for acid-base diagnosis without also examining the
corresponding serum electrolytes. Remember that a serum CO2 out of the normal range always
represents some type of acid-base disorder (barring lab or transcription error), and that serum CO2
may also be normal in the presence of two or more acid-base disorders. Calculate the anion gap
(AG) and if it is elevated, calculate the bicarbonate gap. An AG 20 mEq/L strongly indicates an
anion gap acidosis. If the bicarbonate gap deviates more than ±6 mEq/L there is likely another
acid-base disorder besides AG metabolic acidosis. (See Chapter 7, including Figure 7-2.)
|
|
TIP 2. Single acid-base disorders do not lead to normal blood pH. Although pH can end up in the
normal range (7.35 - 7.45) with single disorders of a mild degree, a truly normal pH with distinctly
abnormal HCO3- and PaCO2 invariably suggests two or more primary disorders. Example: pH
7.40, PaCO2 20 mm Hg, HCO3- 12 mEq/L, in a patient with sepsis. This patient's normal pH
resulted from two co-existing and unstable acid-base disorders: acute respiratory alkalosis and
metabolic acidosis.
|
|
TIP 3.
Simplified rules predict the pH and HCO3- for a given change in PaCO2. If the pH or
HCO3- is higher or lower than expected for the change in PaCO2, the patient probably has a
metabolic acid-base disorder as well. The rules in Table 8-3 show the expected changes in pH and
HCO3- (in mEq/L) for a 10 mm Hg change in PaCO2 from either primary hypoventilation
(respiratory acidosis) or primary hyperventilation (respiratory alkalosis).
|
Table 8-3. Changes in pH and HCO3- for a 10
mm Hg change in PaCO2
PaCO2 in mm Hg, HCO3- in mEq/L
|
ACUTE CHANGE |
CHRONIC CHANGE |
Resp Acidosis
(for PaCO2 up to 70) |
pH down by 0.07
HCO3- up by 1 |
pH down by 0.03
HCO3- up by 3-4 |
Resp Alkalosis
(for PaCO2 down to 20) |
pH up by 0.08
HCO3- down by 2 |
pH up by 0.03
HCO3- down by 5 |
These rules are quite useful in diagnosing a mixed acid-base disorder when there is
respiratory acidosis or respiratory alkalosis. These two conditions of course describe
acute changes in PaCO2, and therefore acute changes in the dissolved CO2 in the blood.
Changes in PaCO2, i.e., in the dissolved fraction of CO2, affect the hydration of
dissolved CO2 with H2O, which is a reversible reaction:
CO2 + H2O <------> H2CO3 <------> H+ + HCO3-.
Acute CO2 retention (i.e., acute respiratory acidosis) drives the hydration reaction
more than normal to the right; as a result, HCO3- increases slightly. Acute CO2
excretion (i.e., acute respiratory alkalosis) drives the hydration reaction more than
normal to the left, and HCO3- decreases slightly. These changes in HCO3- are
instantaneous by virtue of changes in the CO2 hydration reaction, and have nothing to
do with the kidneys or renal compensation. Thus:
a) A normal or slightly low HCO3- in the presence of hypercapnia suggests a
concomitant metabolic acidosis, e.g., pH 7.27, PaCO2 50 mm Hg, HCO3-
22 mEq/L;
b) A normal or slightly elevated HCO3- in the presence of hypocapnia suggests
a concomitant metabolic alkalosis, e.g., pH 7.56, PaCO2 30 mm Hg,
HCO3- 26 mEq/L.
|
TIP 4.
In maximally-compensated metabolic acidosis (which takes about 12-24
hours), the following formula applies:
Expected PaCO2 = (1.5 x serum CO2) + (8 ±2)
A shortcut to this formula is the interesting observation, in maximally compensated
metabolic acidosis, that the numerical value of PaCO2 should be the same (or close
to) the last two digits of arterial pH (Narins 1980). Thus:
Expected PaCO2 = last two digits of pH ± 2
In contrast, the compensation for metabolic alkalosis (by increasing PaCO2) is
highly variable, and in some cases there may be no or minimal compensation.
|
?
For each of the following sets of arterial blood gas values, what is (are) the
likely acid-base disorder(s)?
a) pH 7.28, PaCO2 50 mm Hg, HCO3- 23 mEq/L
b) pH 7.50, PaCO2 33 mm Hg, HCO3- 25 mEq/L
c) pH 7.25, PaCO2 30 mm Hg, HCO3- 14 mEq/L
|
In a), the bicarbonate is lower than expected for acute hypo-ventilation; the patient
has respiratory acidosis and metabolic acidosis. In b), bicarbonate is higher than
expected for acute hyperventilation; the patient has respiratory alkalosis and metabolic
alkalosis. In c), PaCO2 is higher than expected for fully compensated metabolic
acidosis, suggesting a concomitant respiratory disorder or very early metabolic
acidosis.
Always keep in mind that any isolated measurement of pH and PaCO2 can be
explained by two or more co-existing acid-base disorders. Thus, even when blood gas
values fall into one of the 95% confidence bands, the patient may still have a mixed
disorder. Often, the only way to know for sure is by detailed analysis of all the clinical
and laboratory information and close patient follow up.
|
?
Explain the acid-base status of a 35-year-old man admitted to hospital with
pneumonia and the following lab values:
| ARTERIAL BLOOD GASES |
SERUM ELECTROLYTES |
| pH 7.52 |
Na + 145 mEq/L |
| PaCO2 30 mm Hg |
K+ 2.9 mEq/L |
| PaO2 62 mm Hg |
Cl- 98 mEq/L |
|
CO2 21 mEq/L |
His pH and PaCO2 fit into the band of acute respiratory alkalosis. He has moderate
hypoxemia and the blood gas data alone could be explained by acute hyperventilation
due to pneumonia. But the anion gap is elevated at 26 mEq/L, indicating a concomitant
metabolic acidosis. The delta anion gap is 14 mEq/L, giving an expected serum CO2 of
13 mEq/L, and a bicarbonate gap +8 mEq/L. Thus the patient manifests three
separate acid-base disorders: respiratory alkalosis (from pneumonia); metabolic
acidosis (from renal disease); and hypokalemic metabolic alkalosis (from excessive
diuretic therapy). The result of all this acid-base abnormality? Blood gas values that
are indistinguishable from those of simple acute respiratory alkalosis.
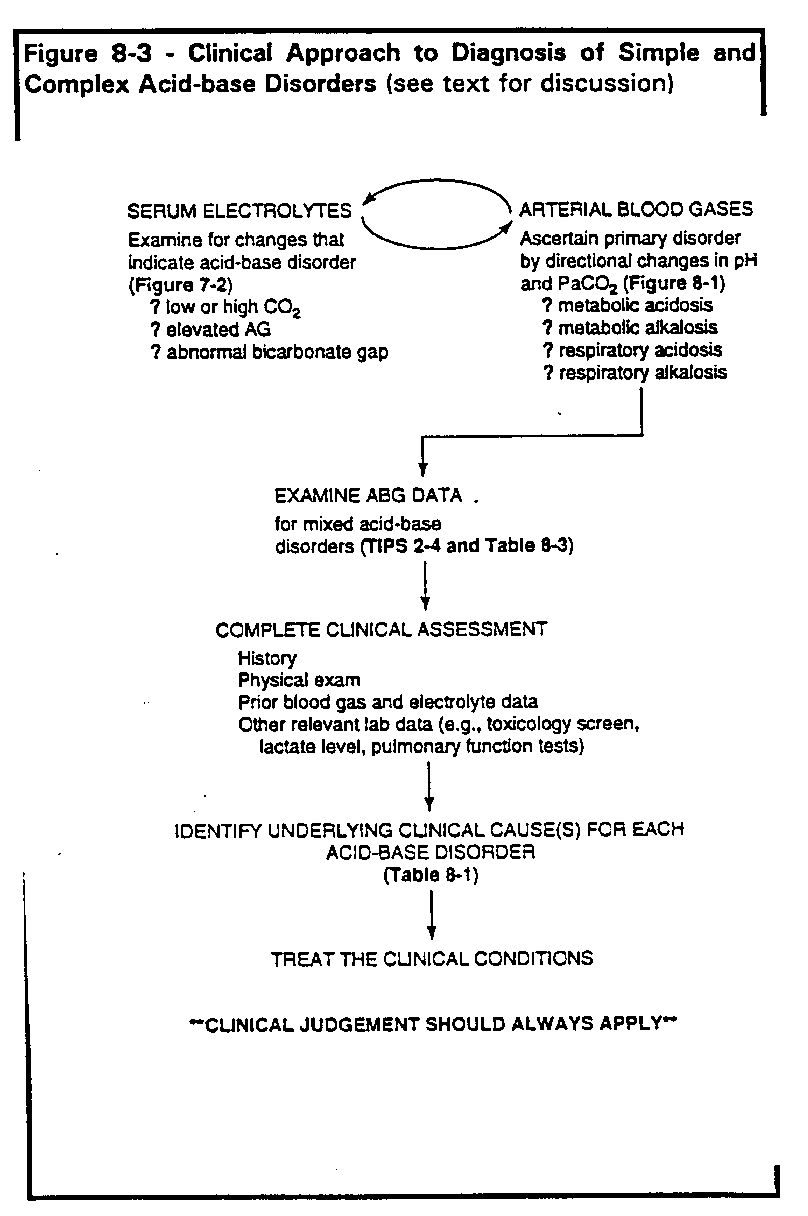
Summary: Clinical and laboratory approach to acid-base diagnosis
Each primary acid-base disorder should be viewed as a physiologic process caused
by a specific clinical condition or disease, not simply as changes in blood gas and
electrolyte values. This view allows for unraveling complex or mixed acid-base
disorders. Points to remember for proper acid-base diagnosis and management can be
summarized as follows (Figure 8-3).
- Determine existence of acid-base disorder from arterial blood gas and/or serum
electrolyte measurements. For electrolytes, follow steps outlined in Figure 7-2.
Check serum CO2 for abnormal value. Calculate AG and, if elevated, calculate
the bicarbonate gap.
- If the measured pH and/or PaCO2 are abnormal, you should be able to discern
at least one primary acid-base disorder from the observed changes
(Table 8-1).
The changes for each primary disorder are straightforward and should be
committed to memory.
- Examine pH, PaCO2 and HCO3- for deviations that may indicate mixed acid-base disorders (TIPS 2 through 4, Table 8-3.)
- Use a full clinical assessment (history, physical exam, other lab data including
previous arterial blood gases and serum electrolytes) to explain each acid-base
disorder (Table 8-1).
- Remember that co-existing clinical conditions may lead to opposing acid-disorders, so that pH can be high when there is an obvious acidosis, or low
when there is an obvious alkalosis.
- Treat the underlying clinical condition(s); this will usually suffice to correct most
acid-base disorders. If there is concern that acidemia or alkalemia is life-threatening, aim toward correcting pH into the range of 7.30-7.52 ([H+] 50-30
nM/L).
- Clinical judgment should always apply. See Pitfalls 12-14 in Chapter 13.
|
Clinical Problem 8-3. A 45-year-old man comes to hospital complaining of dyspnea for
three days. Arterial blood gas reveals pH 7.35, PaCO2 60 mm Hg, PaO2 57 mm Hg,
HCO3- 31 mEq/L. How would you characterize his acid-base status?
|
|
Clinical Problem 8-4. A 53-year-old man initially presents
to the emergency department with the following blood gas values:
| ARTERIAL BLOOD GASES |
| FIO2 .21 |
| PaO2 40 mm Hg |
| PaCO2 50 mm Hg |
| pH 7.51 |
| HCO3- 39 mEq/L |
At this point his acid-base disorder is best characterized as:
a) metabolic alkalosis alone
b) metabolic alkalosis plus respiratory acidosis
c) respiratory acidosis with metabolic compensation
d) can't be certain without more information
He is found to have congestive heart failure and is treated with supplemental oxygen
and diuretics. Three days later he is clinically improved, with pH 7.38, PaCO2 60 mm
Hg, HCO3- 34 mEq/L, and PaO2 73 mm Hg (on FIO2 24%). How would you
characterize his acid-base status now?
|
Clinical Problem 8-5. The following values are found in a
65-year-old patient.
| ARTERIAL BLOOD GASES |
VENOUS BLOOD MEASUREMENTS |
| pH 7.51 |
Na + 155 mEq/L |
| PaCO2 50 mm Hg |
K+ 5.5 mEq/L |
| HCO3- 39 mEq/L |
Cl- 90 mEq/L |
|
CO2 40 mEq/L |
|
BUN 121 mg/dl |
|
Glucose 77 mg/dl |
Which of the following most closely describes this patient's acid-base status?
a) severe metabolic acidosis
b) severe respiratory acidosis
c) respiratory acidosis plus metabolic alkalosis
d) metabolic alkalosis plus metabolic acidosis
e) respiratory acidosis plus respiratory alkalosis
|
Clinical Problem 8-6. A 52-year-old woman has been mechanically ventilated for two
days following a drug overdose. Her arterial blood gas values and electrolytes, stable
for the past 12 hours, show:
| ARTERIAL BLOOD GASES |
VENOUS BLOOD MEASUREMENTS |
| pH 7.45 |
Na + 142 mEq/L |
| PaCO2 25 mm Hg |
K+ 4.0 mEq/L |
|
Cl- 100 mEq/L |
|
CO2 18 mEq/L |
Based on this information, how would you assess her acid-base status?
|
Clinical Problem 8-7. An 18-year-old college student is admitted to the ICU for an acute
asthma attack, after not responding to treatment received in the emergency
department. ABG values (on room air) show: pH 7.46, PaCO2 25 mm Hg, HCO3-
17 mEq/L, PaO2 55 mm Hg, SaO2 87%. Her peak expiratory flow rate is 95 L/min
(25% of predicted value).
Asthma medication is continued. Two hours later she becomes more tired and peak
flow is < 60 L/minute. Blood gas values (on 40% oxygen) now show: pH 7.20,
PaCO2 52 mm Hg, HCO3- 20 mEq/L, PaO2 65 mm Hg. At this point intubation and
mechanical ventilation are considered. What is her acid-base status?
|
|
Clinical Problem 8-8. A 72-year-old man is admitted in shock, with 70 mm Hg systolic
blood pressure. He has a history of chronic obstructive pulmonary disease, and his
baseline ABG is 7.34, PaCO2 68 mm Hg, PaO2 65 mm Hg (on supplemental oxygen),
HCO3- 36 mEq/L. He takes medication for a heart condition. Initial arterial blood gas
results on admission (FIO2 .40) show:
| ARTERIAL BLOOD GASES |
| pH 7.10 |
| PaO2 35 mm Hg |
| PaCO2 70 mm Hg |
| SaO2 58% |
| HCO3- 21 mEq/L |
He is intubated. Repeat blood gases (on the same FIO2) show:
| ARTERIAL BLOOD GASES |
| pH 7.30 |
| PaO2 87 mm Hg |
| PaCO2 40 mm Hg |
| SaO2 98% |
| HCO3- 19 mEq/L |
Assuming his anion gap is elevated at 23 mEq/L, how would you described the
acid-base changes?
|
Clinical Problem 8-9. In review, state whether each of the following statements is true
or false.
a) Metabolic acidosis is always present when the measured serum CO2 changes
acutely from 24 to 21 mEq/L.
b) In acute respiratory acidosis, bicarbonate initially rises because of the reaction of
CO2 with water and the resultant formation of H2CO3.
c) If pH and PaCO2 are both above normal, the calculated bicarbonate must also be
above normal.
d) An abnormal serum CO2 value always indicates an acid-base disorder of some
type.
e) The compensation for chronic elevation of PaCO2 is renal excretion of
bicarbonate.
f) A normal pH with abnormal HCO3- or PaCO2 suggests the presence of two or
more acid-base disorders.
g) A normal serum CO2 value indicates there is no acid-base disorder.
h) Normal arterial blood gas values rule out the presence of an acid-base disorder.
|
END OF SECTION
Lawrence Martin, M.D.
Answers to Problems
Return to Alphabetical Index /
Return to Subject Index
| | | | | |

