We Can't Kill Your Mother! & Other Stories of Intensive Care. Medical and Ethical Challenges in the ICU. Now at Amazon.com in print format (left) and Kindle e-edition (right).10 Common Misconceptions & Errors in Treating COPD (chronic obstructive pulmonary disease)Lawrence Martin, M.D.
|
These 10 common misconceptions & errors in treating COPD are based on many years' experience in pulmonary medicine practice. A companion web site deals with errors in treating asthma. Since the two conditions share similar features, and may occur in the same patient, some of the errors are similar. Note that this list contains misconceptions & errors held by or made by patients and doctors. |
1. Not making the diagnosis
You can't treat if you don't make the diagnosis, and the diagnosis is too often
missed. Any middle aged smoker, and certainly anyone with a history of dyspnea (shortness of
breath) or chronic cough should have spirometry to discern a pattern of air flow obstruction. COPD is generally recognized as being under diagnosed, despite the confirmed 12 million cases in the U.S. alone.
Almost all internal medicine physicians who treat adults have an EKG machine, but most don't have
spirometry, or ready access to spirometry testing. Yet this test alone can uncover people
with "air flow obstruction" and lead to the diagnosis of "chronic air flow obstruction" or
COPD - chronic obstructive pulmonary disease. The consequences of not making the diagnosis
can include:
- less emphasis on smoking cessation
- under-treatment of exacerbations
- worsening of the COPD
The value of spirometry is emphasized in two articles I wrote for a trade magazine distributed to
respiratory therapists:
The Value of Spirometry
in Clinical Practice, Part 1, June 2010 and
Part 2, July 2010.
RT for Decision Makers in Respiratory Care.
- less emphasis on smoking cessation
- under-treatment of exacerbations
- worsening of the COPD
The value of spirometry is emphasized in two articles I wrote for a trade magazine distributed to respiratory therapists: The Value of Spirometry in Clinical Practice, Part 1, June 2010 and Part 2, July 2010. RT for Decision Makers in Respiratory Care.
A comment about chest X-rays and chest CT scans
Ironically, the diagnosis of COPD is sometimes made erroneously by radiologists when they interpret a patient's chest x-ray. I have seen several patients who had a chest x-ray done for a variety of reasons, with the report coming back "COPD." This is medically improper, as the diagnosis can only be made clinically and by spirometry (see ERROR No. 2). What the radiologist sees usually is "hyperinflation" or increase in lung size, something common in young and/or thin people. (An example is shown in the chest x-ray below; the lungs appear larger than normal.) When these mis-diagnosed people have spirometry it is normal, proving that the x-ray interpretation was in error.
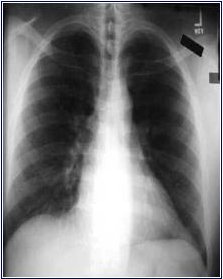
ON THE OTHER HAND, a chest CT scan will often show "emphysema" which is one type of COPD (less common than the other type, which is chronic bronchitis). When the radiologist diagnoses "emphysema" on the chest CT scan, he or she is usually correct. This is because emphysema is really an anatomic diagnosis, and the CT scan gives a good picture of lung anatomy (not true of the plain chest x-ray, however). In the CT scan image below, the dark black areas are "holes" where the lung tissue has disappeared, the hallmark of emphysema. What the radiologist cannot do is assess severity, so that even with a CT scan showing "emphysema" the patient could still have a very wide range of impairment: from NONE (no symptoms or breathing impairment, and therefore no diagnosis of COPD) to SEVERE. Again, the gauge of severity, as well as the actual diagnosis of COPD, depends on the spirometry breathing test.

2. Not checking objective measurement of the patient's air flow obstruction.
Every patient suspected of having COPD should have a breathing test to ascertain the degree of pulmonary impairment. In most cases the test that is needed is 'spirometry', which takes just a few minutes. It requires the patient to inhale fully and then exhale forcefully through a testing device (shown below).
 A patient performing the spirometry test
A patient performing the spirometry test

Above: graphs from a normal spirometry test; left panel, graph of flow vs. volume; right panel, graph of time vs. volume.
Below: examples of spirometry from patients with COPD. On left is flow-volume curve in COPD. The patient's effort in the top half of the graph should cover the dotted line, to make a more triangular tracing. Instead it slopes well below the dotted line, signifying airflow obstruction. The same effort can be graphed as a plot of time vs. volume, as shown on right; in this figure the blue line is normal and the red signifies airways obstruction. The values FEV1 (=forced expiratory value in one second) and FVC (=forced vital capacity) are the principal measurements derived from spirometry. The more abnormal they are, the more severe is the lung impairment (see GOLD criteria, below).


Measuring air flow is analagous to checking a patient's
blood sugar to monitor diabetes - a measurement to determine how bad (or good) the condition is.
Spirometry need not be done often (frequency depending on severity and chronicity of symptoms),
but should be obtained at least once in the course of management.
Spirometry can be a valuable test in diagnosing and managing respiratory
problems, especially COPD and asthma. The following article (in 2 parts) is for respiratory therapists, nurses, physicians and anyone else who may be responsible for ordering and/or interpreting spirometry.
The Value of Spirometry
in Clinical Practice, Part 1, June 2010 and
Part 2, July 2010.
RT for Decision Makers in Respiratory Care.
Classification of severity is based on the
GOLD criteria. GOLD (Global Initiative for Chronic Obstructive Lung Disease) is an international (NIH and WHO) classification that divides COPD
severity into four Stages:
IN PATIENTS WITH FEV-1/FVC <70% absolute:
Classification of severity is based on the
GOLD criteria. GOLD (Global Initiative for Chronic Obstructive Lung Disease) is an international (NIH and WHO) classification that divides COPD
severity into four Stages:
IN PATIENTS WITH FEV-1/FVC <70% absolute:
Stage I -- Mild COPD -- FEV1 >= 80% of predicted
Stage II -- Moderate COPD -- FEV-1 = 50-79% of predicted
Stage III -- Severe COPD -- FEV-1 = 30-49% of predicted
Stage IV -- Very Severe COPD -- FEV-1 <30% normal of predicted or <50% predicted with chronic respiratory failure present
While shortened life span and frequency of "COPD exacerbations" do correlate with GOLD criteria, the system does not take into account other important factors, such as: patient weight; continued smoking; co-morbid condition, especially heart disease; physical conditioning and exercise.
For general audience: Click
here for Wikipedia article on COPD, which includes discussion of GOLD criteria.
For medical professionals: Click here to download teaching slides on GOLD criteria.
3. Not emphasizing smoking cessation.
Too often physicians don't spend enough time on patients with lung disease
to get them to quit smoking. It's got to be more than 'Joe, you should quit, or cut down.",
Smoking cessation in a patient with COPD (or asthma) has to be the focus of treatment,
in some ways more important than prescribing medication. A few well-placed and
smartly emphasized words from a physician can have a powerful effect on patients. At the
same time, language couched in phrases like 'you should quit' or 'it would be a good idea
to cut down' give subtle message that maybe its not so bad or so important to really stop.
And that's not true. I won't stop making the point until the patient knows I'm serious,
knows that smoking cessation is center stage in his/her therapy.
At the same time patients have responsibility for their health care and shouldn't
need a physician's admonishments to quit.
I see many patients who continue to smoke while complaining
of cough or shortness of breath. Sure, they admit to
being addicted, or "I just can't stop", but there is still no
excuse. Smoking could either be the direct cause of wheezing
and shortness of breath (what we generally call "acute bronchitis").
Also, smoking greatly retards
recovery, since cigarette smoke impairs clearing of mucus from
the lungs. I tell my patients it's like complaining of
a headache while banging your head with a hammer. Duh!
4. Thinking a written prescription for a COPD inhaler means
the patient knows how to use it: The device itself.
Stage III -- Severe COPD -- FEV-1 = 30-49% of predicted
Stage IV -- Very Severe COPD -- FEV-1 <30% normal of predicted or <50% predicted with chronic respiratory failure present
While shortened life span and frequency of "COPD exacerbations" do correlate with GOLD criteria, the system does not take into account other important factors, such as: patient weight; continued smoking; co-morbid condition, especially heart disease; physical conditioning and exercise.
For general audience: Click
here for Wikipedia article on COPD, which includes discussion of GOLD criteria.
For medical professionals: Click here to download teaching slides on GOLD criteria.
3. Not emphasizing smoking cessation.
Too often physicians don't spend enough time on patients with lung disease
to get them to quit smoking. It's got to be more than 'Joe, you should quit, or cut down.",
Smoking cessation in a patient with COPD (or asthma) has to be the focus of treatment,
in some ways more important than prescribing medication. A few well-placed and
smartly emphasized words from a physician can have a powerful effect on patients. At the
same time, language couched in phrases like 'you should quit' or 'it would be a good idea
to cut down' give subtle message that maybe its not so bad or so important to really stop.
And that's not true. I won't stop making the point until the patient knows I'm serious,
knows that smoking cessation is center stage in his/her therapy.
At the same time patients have responsibility for their health care and shouldn't
need a physician's admonishments to quit.
I see many patients who continue to smoke while complaining
of cough or shortness of breath. Sure, they admit to
being addicted, or "I just can't stop", but there is still no
excuse. Smoking could either be the direct cause of wheezing
and shortness of breath (what we generally call "acute bronchitis").
Also, smoking greatly retards
recovery, since cigarette smoke impairs clearing of mucus from
the lungs. I tell my patients it's like complaining of
a headache while banging your head with a hammer. Duh!
4. Thinking a written prescription for a COPD inhaler means
the patient knows how to use it: The device itself.
While shortened life span and frequency of "COPD exacerbations" do correlate with GOLD criteria, the system does not take into account other important factors, such as: patient weight; continued smoking; co-morbid condition, especially heart disease; physical conditioning and exercise.
For general audience: Click
here for Wikipedia article on COPD, which includes discussion of GOLD criteria.
For medical professionals: Click here to download teaching slides on GOLD criteria.
3. Not emphasizing smoking cessation.
Too often physicians don't spend enough time on patients with lung disease
to get them to quit smoking. It's got to be more than 'Joe, you should quit, or cut down.",
Smoking cessation in a patient with COPD (or asthma) has to be the focus of treatment,
in some ways more important than prescribing medication. A few well-placed and
smartly emphasized words from a physician can have a powerful effect on patients. At the
same time, language couched in phrases like 'you should quit' or 'it would be a good idea
to cut down' give subtle message that maybe its not so bad or so important to really stop.
And that's not true. I won't stop making the point until the patient knows I'm serious,
knows that smoking cessation is center stage in his/her therapy.
At the same time patients have responsibility for their health care and shouldn't
need a physician's admonishments to quit.
I see many patients who continue to smoke while complaining
of cough or shortness of breath. Sure, they admit to
being addicted, or "I just can't stop", but there is still no
excuse. Smoking could either be the direct cause of wheezing
and shortness of breath (what we generally call "acute bronchitis").
Also, smoking greatly retards
recovery, since cigarette smoke impairs clearing of mucus from
the lungs. I tell my patients it's like complaining of
a headache while banging your head with a hammer. Duh!
4. Thinking a written prescription for a COPD inhaler means
the patient knows how to use it: The device itself.
3. Not emphasizing smoking cessation.
Too often physicians don't spend enough time on patients with lung disease
to get them to quit smoking. It's got to be more than 'Joe, you should quit, or cut down.",
Smoking cessation in a patient with COPD (or asthma) has to be the focus of treatment,
in some ways more important than prescribing medication. A few well-placed and
smartly emphasized words from a physician can have a powerful effect on patients. At the
same time, language couched in phrases like 'you should quit' or 'it would be a good idea
to cut down' give subtle message that maybe its not so bad or so important to really stop.
And that's not true. I won't stop making the point until the patient knows I'm serious,
knows that smoking cessation is center stage in his/her therapy.
At the same time patients have responsibility for their health care and shouldn't
need a physician's admonishments to quit.
I see many patients who continue to smoke while complaining
of cough or shortness of breath. Sure, they admit to
being addicted, or "I just can't stop", but there is still no
excuse. Smoking could either be the direct cause of wheezing
and shortness of breath (what we generally call "acute bronchitis").
Also, smoking greatly retards
recovery, since cigarette smoke impairs clearing of mucus from
the lungs. I tell my patients it's like complaining of
a headache while banging your head with a hammer. Duh!
4. Thinking a written prescription for a COPD inhaler means
the patient knows how to use it: The device itself.
4. Thinking a written prescription for a COPD inhaler means the patient knows how to use it: The device itself.
There are many different kinds of inhalers for COPD on the market. There are also algorithms for their use in COPD, with recommendations generally based on stages of COPD severity. One relatively simple algorithm is shown below, from Pharmacologic therapy of chronic obstructive pulmonary disease, by Dr. Sabeer Abdool-Gaffar.

In this algorithm:
- SABA = short-acting beta2-agonist (e.g., albuterol, brand names
(ProAir, Proventil, Ventolin, Xopenex)
- SAMA = short acting muscarinic antagonist (ipratropium, brand name Atrovent)
- LABA = long-acting beta2-agonist (e.g., formoterol, brand name Foradil; salmeterol, brand name Serevent)
- LAMA = long acting muscarinic antagonist (tiotropium, brand name Spiriva; aclidinium, brand name Tudorza Pressair)
- ICS = inhaled corticosteroid (e.g., beclomethasone, brand name QVAR; budesonide, brand name Pulmicort; mometasone, brand name Asmanex)
- Theophylline = dimethylxanthine, similar to caffeine; a non-selective phosphodiesterase inhibitor, it increases intracellular cAMP to promote bronchodilation. It is given in pill or liquid form, so doesn't contribute to inhaler confusion. However, theophylline is not very effective and can cause significant side effects, so today the drug is seldom used in treating COPD.
Unfortunately, stepped algorithms for use of inhalers, when coupled with a plethora
of inhaler types, often leads to CONFUSION.
Algorithms + inhalers =
CONFUSION
A physician may prescribe the correct inhaler or inhalers, but that is of no value
unless the patient already knows how to use it or is shown how with a demonstration. None of the inhalers is intuitive as to how to use, in contrast to a pill or tablet which
must merely be swallowed to be effective. For each inhaler, the patient must make some maneuver which, if not done correctly, means the medication is not inhaled properly or in
sufficient amount.
Mis-use of inhalers is a major and well-documented
problem that occurs across the entire spectrum of patients. The problem
stems from the plethora of inhaler types (lack of standardization),
their inherent complexity (relative to swallowing a pill),
and lack of training about how to use them among both caregivers and
patients.

If there was just one or two types of inhaler, health care providers
(and by extension, their patients) would become very famililar with them
and there would be less confusion than currently exists. But in fact there
are several different types of inhalers for asthma and COPD. The poster
above displays only a partial list of inhalers for asthma and COPD
(they are the same for either condition).
The treatment algorithm shown above classifies inhalers by pharmacologic
activity, i.e., how the drugs work to open up the airways.
However, they can also be classified by the physical type of inhaler.
The device itself can make or break the drug's efficacy for the patient.
The three broad physical types of inhalers are 1) pressurized metered dose
inhalers (pMDIs), 2) dry powder inhalers (DPIs) and 3) the newest,
Respimat "soft mist" inhaler.
PRESSURIZED METERED DOSE INHALERS (pMDIs)
These come with a propellant to deliver the drug as a mist. You can
see the mist when the cannister button is pressed down into its plastic housing.

pMDIs deliver (under pressure) a spray of medication from the mouthpiece.
(Though the MDI delivers the drug as a fine mist, the device is very different from
the Respimat "soft mist" inhaler; the Respimat does not use
a propellant and is technically not a 'metered dose inhaler'.)
The MDI mist seen when you press on the MDI cannister must be inhaled to be
effective; thus the paient's lips shouild be on or very close to the mouthpiece
at the time of delivery (see picture below).
The patient must inhale deeply and at the right time in order to get all of
the medication delivered into the lungs.

pMDIs are the most common type of inhaler in the United States; most of the inhalers
shown on the poster above are pMDIs. Short acting bronchodilator medication
is typically delivered via an MDI. To use this device
you must inhale immediately after pressing the canister down
into the plastic housing, as shown. This maneuver requires
some coordination: squeezing the fingers together while
making a deep inhalation with the device held tightly
between your lips. Some people do it well, but others actuall
exhale when they press down on the canister, so the medication doesn't enter the lungs.
Click here for information on how to use the pMDI
What about spacers for metered dose inhalers?
Spacers have long been used with pMDIs to make it easier for
patients to inhale the medication. Another name for spacers is
aerosol-holding chambers. Two examples are shown below: a rigid
plastic spacer on the left and a collapsible spacer.
The pMDI inserts in one part of the spacer and
the patient inhales from a chamber that holds the medication.
When the pMDI is compressed the medication aerosol
enters the spacer and the patient can breathe normally via a mouthpiece
without the need to closely coordinate inspiration with medication release.
There are many different types of spacers available.


Pros: Spacers makes it easier to inhale the medication, helping assure that
it enters the lungs and not the room environment or just the back of the throat.
The Asthma Society of Canada recommends that anyone using a puffer consider
a spacer.
Cons: They are bulky to carry around, and often not availble when needed
(women can put them in a purse; not so for men). The aerosol medication
can adhere to the chamber wall, lessening the amount available for treatment.
Spacers need to be cleaned or replaced, adding expense to treatment regimen.
Bottom Line: If the pMDI is used as intended, there should be no need for a spacer.
They seem to be most useful for children needing a pMDI. Only a small minority of
adults regularly use a spacer with their pMDI.
DRY POWDERED INHALERS (DPIs)
With DPIs the patient's breath (rather than hand action) actuates delivery of the
medication. You cannot see the spray because the only way to get the medication is to
inhale it from the mouthpiece. Within the DPI category, there are two broad types:
DPI Type 1): the medication is contained within the inhaler device at all times,
until inhaled, or
DPI Type 2): the medication comes in a separate capsule that must be placed
into the inhaler device at the time of use.
One study that received wide publicity showed that up to 1/3 of patients use
DPIs incorrectly. The error rate increased with patient's age, and
correlated with lack of instruction to the patient.
DPI Type 1. Medication is contained within the device
- Turbuhaler (also known as Flexhaler). Several medications are
delivered via the tubuhaler, including the steroid
Pulmicort
(shown below; the manufacturer, Astra Zeneca calls their device a "Flexhaler,"
just another name for turbuhaler). The turbuhaler requires you to
twist the dark cap shown at the bottom in order to activate the
next inhalation. The turbuhaler (flexhaler)
eliminates the type of coordinated effort needed for
traditional MDIs, since once activated all you have to do
is inhale from the mouthpiece. However, the bottom cap can twist
both right and left, and it's not obvious which
way activates the flexhaler. Thus some patients twist
it so as to close the chamber, preventing delivery
of medication when they inhale. For instructions on how to use
the turbuhaler/flexhaler, see:
National Jewish Hospitals web site
Asthma Society of Canada web site

- Twisthaler is another plastic device that contains dry powdered medication.
Like the turbuhaler, the bottom cap (in this case pink; see picture below) must
be twisted to prepare the medication for delivery by breath inhalation.
Asmanex, an inhaled steroid, is delivered via a twisthaler (see below). When
you inhale (after twisting the cap), the twisthaler automatically releases the medication.
As with the other DPIs, when inhaled correctly the medication is delivered
properly. For instructions on how to use the twisthaler, see
National Jewish Hospital web site
- Diskus inhaler (see below).
Advair and
Serevent come in
this 'flying saucer' inhaler. The Diskus inhaler
requires sliding two different levers on the side, one to
activate the medicine for delivery and the other
to open the channel so the medication can be inhaled.
Each lever is accompanied by a 'click' and the patient
is suppose to make sure there are "two clicks" each time
they prepare to use the inhaler. However,
many patients only do one click,which means
the inhaler may actually be closed when they breathe
in, so no medicine is delivered. For instructions on
how to use the diskus inhaler, see:
National Jewish Hospital web site
Asthma Society of Canada web site

- Diskhaler (see below).
The diskhaler is not available in the United States, but is used in
Canada, England and other countries.
Serevent
is one of the medications that comes in a diskhaler.
The diskhaler is somewhat of a hybrid between the two types of DPI,
in that the medication comes prepackaged on a disk, with
each disk containing 8 separate doses. The disk
is inserted into the inhaler, and only needs replacement after the 8
doses are used up. The patient need never touch the medication itself.
For instructions on how to use the diskus inhaler, see:
Asthma Society of Canada web site

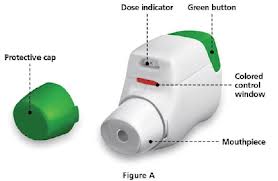
- Pressair inhaler (see below).
The drug Tudorza comes in
the latest type of dry powder inhaler called "Pressair."
The full name of the drug is "Tudorza Pressair," with Tudorza being the drug
and Pressair being the type of inhaler. Tudorza was approved by the FDA
in July 2012 for treatment of bronchospasm associated with
chronic obstructive pulmonary disease or COPD. (Technically Tudorza
Pressair is approved only for COPD, not asthma. However, many patients with
asthma
also have chronic lung disease and are prescribed Tudorza.). The drug is inside the inhaler, ready to use as soon as the patient presses the large green button at the back of the inhaler; this action changes a small window in the front from red to green (see photo). The patient then inhales from the mouthpiece with enough effort to change the window color back to red; this action is also signified by a reassuring 'click'. Note that gentle inhaling won't do it. If the patient does not hear the 'click', the window does not change from green to red and the patient did not receive the drug. (Studies have shown that even patients with severe COPD can easily inhale with enough force to get the drug.) Tudorza is designed to be inhaled twice a day (one inhalation each time). Details of how to use Tudorza can be found in the
Tudorza drug information (scroll down for drawings).
Unfortunately, stepped algorithms for use of inhalers, when coupled with a plethora of inhaler types, often leads to CONFUSION.
Algorithms + inhalers =
CONFUSION
A physician may prescribe the correct inhaler or inhalers, but that is of no value unless the patient already knows how to use it or is shown how with a demonstration. None of the inhalers is intuitive as to how to use, in contrast to a pill or tablet which must merely be swallowed to be effective. For each inhaler, the patient must make some maneuver which, if not done correctly, means the medication is not inhaled properly or in sufficient amount. Mis-use of inhalers is a major and well-documented problem that occurs across the entire spectrum of patients. The problem stems from the plethora of inhaler types (lack of standardization), their inherent complexity (relative to swallowing a pill), and lack of training about how to use them among both caregivers and patients.

If there was just one or two types of inhaler, health care providers (and by extension, their patients) would become very famililar with them and there would be less confusion than currently exists. But in fact there are several different types of inhalers for asthma and COPD. The poster above displays only a partial list of inhalers for asthma and COPD (they are the same for either condition).
The treatment algorithm shown above classifies inhalers by pharmacologic activity, i.e., how the drugs work to open up the airways. However, they can also be classified by the physical type of inhaler. The device itself can make or break the drug's efficacy for the patient. The three broad physical types of inhalers are 1) pressurized metered dose inhalers (pMDIs), 2) dry powder inhalers (DPIs) and 3) the newest, Respimat "soft mist" inhaler.
PRESSURIZED METERED DOSE INHALERS (pMDIs)
These come with a propellant to deliver the drug as a mist. You can
see the mist when the cannister button is pressed down into its plastic housing.

pMDIs deliver (under pressure) a spray of medication from the mouthpiece.
(Though the MDI delivers the drug as a fine mist, the device is very different from
the Respimat "soft mist" inhaler; the Respimat does not use
a propellant and is technically not a 'metered dose inhaler'.)
The MDI mist seen when you press on the MDI cannister must be inhaled to be
effective; thus the paient's lips shouild be on or very close to the mouthpiece
at the time of delivery (see picture below).
The patient must inhale deeply and at the right time in order to get all of
the medication delivered into the lungs.

pMDIs are the most common type of inhaler in the United States; most of the inhalers
shown on the poster above are pMDIs. Short acting bronchodilator medication
is typically delivered via an MDI. To use this device
you must inhale immediately after pressing the canister down
into the plastic housing, as shown. This maneuver requires
some coordination: squeezing the fingers together while
making a deep inhalation with the device held tightly
between your lips. Some people do it well, but others actuall
exhale when they press down on the canister, so the medication doesn't enter the lungs.
Click here for information on how to use the pMDI
What about spacers for metered dose inhalers?Spacers have long been used with pMDIs to make it easier for patients to inhale the medication. Another name for spacers is aerosol-holding chambers. Two examples are shown below: a rigid plastic spacer on the left and a collapsible spacer. The pMDI inserts in one part of the spacer and the patient inhales from a chamber that holds the medication. When the pMDI is compressed the medication aerosol enters the spacer and the patient can breathe normally via a mouthpiece without the need to closely coordinate inspiration with medication release. There are many different types of spacers available. 
Pros: Spacers makes it easier to inhale the medication, helping assure that
it enters the lungs and not the room environment or just the back of the throat.
The Asthma Society of Canada recommends that anyone using a puffer consider
a spacer.
|
DRY POWDERED INHALERS (DPIs)
With DPIs the patient's breath (rather than hand action) actuates delivery of the
medication. You cannot see the spray because the only way to get the medication is to
inhale it from the mouthpiece. Within the DPI category, there are two broad types:
DPI Type 1): the medication is contained within the inhaler device at all times,
until inhaled, or
DPI Type 2): the medication comes in a separate capsule that must be placed
into the inhaler device at the time of use.
One study that received wide publicity showed that up to 1/3 of patients use
DPIs incorrectly. The error rate increased with patient's age, and
correlated with lack of instruction to the patient.
DPI Type 1): the medication is contained within the inhaler device at all times,
until inhaled, or
DPI Type 2): the medication comes in a separate capsule that must be placed
into the inhaler device at the time of use.
DPI Type 1. Medication is contained within the device
- Turbuhaler (also known as Flexhaler). Several medications are
delivered via the tubuhaler, including the steroid
Pulmicort
(shown below; the manufacturer, Astra Zeneca calls their device a "Flexhaler,"
just another name for turbuhaler). The turbuhaler requires you to
twist the dark cap shown at the bottom in order to activate the
next inhalation. The turbuhaler (flexhaler)
eliminates the type of coordinated effort needed for
traditional MDIs, since once activated all you have to do
is inhale from the mouthpiece. However, the bottom cap can twist
both right and left, and it's not obvious which
way activates the flexhaler. Thus some patients twist
it so as to close the chamber, preventing delivery
of medication when they inhale. For instructions on how to use
the turbuhaler/flexhaler, see:
National Jewish Hospitals web site
Asthma Society of Canada web site
- Twisthaler is another plastic device that contains dry powdered medication.
Like the turbuhaler, the bottom cap (in this case pink; see picture below) must
be twisted to prepare the medication for delivery by breath inhalation.
Asmanex, an inhaled steroid, is delivered via a twisthaler (see below). When
you inhale (after twisting the cap), the twisthaler automatically releases the medication.
As with the other DPIs, when inhaled correctly the medication is delivered
properly. For instructions on how to use the twisthaler, see
National Jewish Hospital web site

- Diskus inhaler (see below).
Advair and
Serevent come in
this 'flying saucer' inhaler. The Diskus inhaler
requires sliding two different levers on the side, one to
activate the medicine for delivery and the other
to open the channel so the medication can be inhaled.
Each lever is accompanied by a 'click' and the patient
is suppose to make sure there are "two clicks" each time
they prepare to use the inhaler. However,
many patients only do one click,which means
the inhaler may actually be closed when they breathe
in, so no medicine is delivered. For instructions on
how to use the diskus inhaler, see:
National Jewish Hospital web site
Asthma Society of Canada web site

- Diskhaler (see below).
The diskhaler is not available in the United States, but is used in
Canada, England and other countries.
Serevent
is one of the medications that comes in a diskhaler.
The diskhaler is somewhat of a hybrid between the two types of DPI,
in that the medication comes prepackaged on a disk, with
each disk containing 8 separate doses. The disk
is inserted into the inhaler, and only needs replacement after the 8
doses are used up. The patient need never touch the medication itself.
For instructions on how to use the diskus inhaler, see:
Asthma Society of Canada web site

- Pressair inhaler (see below).
The drug Tudorza comes in
the latest type of dry powder inhaler called "Pressair."
The full name of the drug is "Tudorza Pressair," with Tudorza being the drug
and Pressair being the type of inhaler. Tudorza was approved by the FDA
in July 2012 for treatment of bronchospasm associated with
chronic obstructive pulmonary disease or COPD. (Technically Tudorza
Pressair is approved only for COPD, not asthma. However, many patients with
asthma
also have chronic lung disease and are prescribed Tudorza.). The drug is inside the inhaler, ready to use as soon as the patient presses the large green button at the back of the inhaler; this action changes a small window in the front from red to green (see photo). The patient then inhales from the mouthpiece with enough effort to change the window color back to red; this action is also signified by a reassuring 'click'. Note that gentle inhaling won't do it. If the patient does not hear the 'click', the window does not change from green to red and the patient did not receive the drug. (Studies have shown that even patients with severe COPD can easily inhale with enough force to get the drug.) Tudorza is designed to be inhaled twice a day (one inhalation each time). Details of how to use Tudorza can be found in the
Tudorza drug information (scroll down for drawings).

DPI Type 2.
Medication is separate from the inhaler, in a capsule
- Handihaler (see below). The widely used drug
Spiriva
(tiotropium bromide) is delivered in this device. (Technically Spiriva
is for chronic obstructive pulmonary disease, not asthma. However, many patients
with"asthma" also have chronic lung disease and are prescribed Spiriva.)
With the handihaler (as with the aerosolizer, below), the
actual medication resides apart from the device,
in capsules that are individually packaged.
The patient must: retrieve the capsule from its
wrapping, place it into the chamber of the handihaler,
close the chamber with a cap, then pierce the capsule by
pushing hard on a lever to the side of the handihaler. It's
a lot of steps! Furthermore, there are anecdotal reports of patients
who swallow the capsule instead of putting into the
chamber for puncture. The drug works well IF the patient is
instructed how to use the device and can perform the
required maneuvers. Without adequate instruction,
proper use is unlikely.For instructions on how to use the handihaler, see
National Jewish Hospital web site.

- Aerolizer. Like the handihaler (discussed
above), the aerolizer consists of a plastic device to
inhale medication. A capsule of powdered medication is placed
in the device and its delivery is breath activated.
Foradil,
a long acting bronchodilator, comes in this device (shown below).
For instructions on how to use
the aerolizer, see
National Jewish Hospital web site.

PROPELLANT-FREE "SOFT MIST" INHALERS
This is the latest type of inhaler for asthma and COPD. At this writing there is only one soft mist inhaler
marketed in the United States,
Combivent Respimat, shown in the photo.
 Combivent has long been available as an MDI, with CFC (chlorofluorocarbon) as
the propellant. The
Respimat device does away with the propellant and delivers
the drug as a fine mist. The Respimat is not an MDI, and is a third type of
inhaler device. Why the change in delivery system? According to the drug company's web site:
Combivent has long been available as an MDI, with CFC (chlorofluorocarbon) as
the propellant. The
Respimat device does away with the propellant and delivers
the drug as a fine mist. The Respimat is not an MDI, and is a third type of
inhaler device. Why the change in delivery system? According to the drug company's web site:
Under the Clean Air Act, the Food and Drug Administration (FDA) has ordered products containing certain propellants, including COMBIVENT MDI, to be removed from the market. COMBIVENT RESPIMAT does not contain any of these harmful propellants and uses a spring mechanism to release the medication. Supplies of COMBIVENT MDI may run out in the second quarter of 2013. For more information on the Clean Air Act and CFCs, see this information from the Food and Drug Administration.
NOTE: Combivent is marketed for COPD, but many patients "with asthma" use it as well.
Unlike the FDA, physicians can't and don't strictly categorize patients with airway obstruction
into "COPD" and "asthma." In adult patients the conditions frequently overlap.
As to the Respimat delivery system, it is anticipated that other drugs will
soon be released using the device. As with ALL inhalers, its use is
not intuitive, and patients should be shown at least once how to use the device. This
can be done by the physician, nurse, medical assistant or pharmacist.
The company gives placebo devices to physicians for demonstration purposes.
NOTE: When these inhalers -- pMDIs, DPIs, Respimat "soft mist" -- are tested in drug
studies there is virtually unlimited support and follow up to assure
the study patients use them correctly. This support is funded
by the drug companies, who obviously want to know if the medication
is effective, so they spend whatever it takes to make sure the enrolled patients
are properly instructed and know how to use the inhalers. That level of
education and support for inhaler use is seldom available
in clinical practice, resulting in discrepancy between the
effectiveness of the drug in published studies versus the real world.
The medication itself may be good but the delivery system is complicated and prone
to mis-use; as a result, improperly-used inhalers are often the
'weak link' in treating the patient's lung disease.
5. Thinking a written prescription for a COPD inhaler means
the patient knows when to use it: The drug's purpose.
For treatment purposes all inhalers for COPD & asthma fall into one of
two broad categories:
a) to provide quick relief ('rescue inhalers') and
b) to improve chronic symptoms and prevent flareups ('maintenance inhalers').
NOTE: When these inhalers -- pMDIs, DPIs, Respimat "soft mist" -- are tested in drug studies there is virtually unlimited support and follow up to assure the study patients use them correctly. This support is funded by the drug companies, who obviously want to know if the medication is effective, so they spend whatever it takes to make sure the enrolled patients are properly instructed and know how to use the inhalers. That level of education and support for inhaler use is seldom available in clinical practice, resulting in discrepancy between the effectiveness of the drug in published studies versus the real world. The medication itself may be good but the delivery system is complicated and prone to mis-use; as a result, improperly-used inhalers are often the 'weak link' in treating the patient's lung disease. |
5. Thinking a written prescription for a COPD inhaler means the patient knows when to use it: The drug's purpose.
For treatment purposes all inhalers for COPD & asthma fall into one of
two broad categories:
a) to provide quick relief ('rescue inhalers') and
b) to improve chronic symptoms and prevent flareups ('maintenance inhalers').
Examples of rescue inhalers are albuterol (brand names Proventil HFA, ProAir HFA, Ventolin HFA) and ipratropium bromide (brand name Atrovent). Combivent contains a combination of albuterol and ipratropium bromide. Maintenance inhalers include any inhaled steroid (IS), either alone (brand names Azmacort, Qvar, Pulmicort, etc.) or in combination with a 'long acting bronchodilator' (LABD; brand names Symbicort, Advair).
PROBLEM: The SAME type of delivery device (size, shape,
mechanism of action) is commonly used for both
rescue and maintenance inhalers. For example, as shown below,
ProAir HFA (a rescue inhaler, on left) and
Symbicort (a maintenance inhaler, on right) both come packaged as pressurized metered dose inhalers, and
both are deep red in color. There is nothing intuitive about this.
For a patient who may have both inhalers (quite common), and who
becomes short of breath, it is all too easy to forget which is which.


This confusing situation happens often, even when the rescue and maintenance inhalers are of different color. The root problem is lack of standardization among inhalers, with unclear labeling to distinguish between rescue and maintenance inhalers. A contributing cause is lack of proper education for both the caregivers and their patients. All too often proper instructions were not given when the drug was first prescribed. And even when they are provided, patients sometimes don't really understand, or they forget. Either way, having similar inhalers for different purposes is an invitation to error. (This was less likely to be a problem when the drug was studied by the drug companies; see NOTE above, under 'DPI Type 2'.)
The problem is compounded when patients are on multiple inhalers, eg, Proventil for rescue, Advair and Spiriva for maintenance. That's three separate devices with two different purposes -- easy for the patient to get confused. (Pills and capsules come in many colors and sizes, but they are all swallowed the same way.) What's needed is a universal delivery device for all inhalers, with perhaps just two colors: red for rescue drugs and green for maintenance drugs.
Anyone with clinical interest in the inhaler problems discussed above (Errors 1 & 2) should definitely read Problems With Inhaler Use: A Call for Improved Clinician and Patient Education, by James B. Fink and Bruck K. Rubin (Respiratory Care, Sept 2005, Vol 50, No. 10, pages 1360-75).
6. Over-using or under-using oxygen therapy.

Oxygen is a widely used 'drug' for patients with lung disease. Unfortunately
it is both over used and under used. There are in fact strict Medicare criteria for
when to prescribe oxygen, and these same criteria are generally followed by health insurance
companies. For example, a patient's "oxygen saturation" must be 88% or lower at rest (93% or higher
is normal if you are at sea level).
Oxygen by prescription is called "supplemental oxygen," since it supplements the oxygen readily
available in the air around us. Many patients prescribed supplemental oxygen refuse to use it,
even though it would help, such as when there is low oxygen saturation during sleep.
Conversely, many patients prescribed oxygen when their oxygen saturation was low no longer need
it (because they have improved), but still resort to using it "when I feel I need it."
The need for oxygen cannot be determined by how one feels, since there are other reasons for shortness of breath besides low oxygen level. In fact, most of the time a feeling of shortness of breath is not from low oxygen levels. Whether or not a patient needs oxygen and can benefit is best determined by direct measurement of oxygen saturation in specific situations (such as with walking or while sleeping).
Oxygen saturation measurement is done with a pulse oximeter, which is now widely available. More and more, patients on oxygen therapy are purchasing an oximeter (from the internet, including amazon.com, or from medical supply stores). In the figure of an oximeter shown below, the number 97 is the oxygen saturation the number 72 is the heart rate; both are normal. A formal report of this information would read: "Oxygen saturation = 97%, heart rate = 72 beats/minute."

The need for oxygen cannot be determined by how one feels, since there are other reasons for shortness of breath besides low oxygen level. In fact, most of the time a feeling of shortness of breath is not from low oxygen levels. Whether or not a patient needs oxygen and can benefit is best determined by direct measurement of oxygen saturation in specific situations (such as with walking or while sleeping).
Oxygen saturation measurement is done with a pulse oximeter, which is now widely available. More and more, patients on oxygen therapy are purchasing an oximeter (from the internet, including amazon.com, or from medical supply stores). In the figure of an oximeter shown below, the number 97 is the oxygen saturation the number 72 is the heart rate; both are normal. A formal report of this information would read: "Oxygen saturation = 97%, heart rate = 72 beats/minute."


I do not ask patients to buy an oximeter, but I don't discourage it either. If you do purchase a pulse oximeter, make sure you discuss its proper use (for your situation) with your physician.
7. Fear of prescribing oral steroids.
Doctors are often reluctant to prescribe oral steroid medication (prednisone, methylprednisolone),
yet many times it is the only drug that will effectively treat the patient's
COPD exacerbation. Instead, all too often physicians prescribe an
inhaled steroid or long acting bronchodilator or combination IS+LABD. These drugs (non-generic and all expensive) have a definite role in COPD, but not in treating the patient whose symptoms are acute, progressive or interfering with daily activity.
Oral prednisone has potential serious side effects when take long term. Taken short term, which I define as less than two weeks, the drug causes few if any side effects. Furthermore, a short burst of prednisone can often obviate hospitalization for a "COPD exacerbation." If hospitalized for this condition the treatment will usually involve a much higher does of steroids than might have prevented the flare up in the first place.
There is no "right dose" of prednisone for a COPD exacerbation. I usually prescribe the
following for adults:
Prednisone 20 mg tablets:
One three times a day for 3 days, followed by
One twice a day for 3 days, followed by
One a day for 5 days, total 20 tablets over 11 days.
8. Missing the diagnosis of COPD because of
"clear lung fields" on exam.
A patient can have clear lungs if the exam is done only during quiet breathing. The examiner places the stethoscope over the lungs and pronounces them 'clear - no wheezing.' In fact wheezing may be heard, but only after a deep breath followed by a forceful exhalation. Here the problem is simply an inexperienced care giver (physician, nurse practioner or other health aide). Many asthmatics and COPD patients with 'clear lung fields' on exam in fact have bad lungs, with wheezes heard only at
the end of a forced exhalation.
9. Over-using antibiotics to treat COPD exacerbations.
Antibiotics are commonly used in treating COPD, but often bacterial
infection is not the problem, or rather using an antibiotic alone won't fix
the problem. The most common triggers of a COPD exacerbation are probably viral
infections, but all physicians use antibiotics in the initial phase. This is
accepted practice, but again, additional medication is usually needed: invariably bronchodilators and steroids.
10. Not considering environmental factors in managing COPD.
Occupationally-related lung disease
is a common problem, and should always
be considered when evaluating an adult with wheezing or shortness of breath that
is not from heart disease. 2nd hand smoke at home or the workplace doesn't typically cause
COPD, but can aggravate the condition. The work environment can cause chronic, low level wheezing and shortness of breath which, over time, can end up as COPD. While treatment is usually the same regardless of precipitating causes (and smoking is by far the most common),
if triggers can be identified the patient MUST avoid them. This may be
difficult if one's livelihood is causing the problem.
Books About COPD -- for a General Audience
(With links to Amazon.com. Books listed in reverse
order of publication)
Live Your Life with COPD - 52 Weeks of Health, Happiness and Hope.
Jane M. Martin, Infinity Publishing, 2011.
The Complete Guide to Understanding and Living with COPD: From a COPDer's Perspective. R.D. Martin, CreateSpace, 2010.
Positive Options for Living with COPD: Self-Help and Treatment for Chronic Obstructive Pulmonary Disease, Teri Allen, Hunter House, 2010.
COPD for Dummies, Kevin Feiner, MD, Meg Scheider, Books for Dummies, 2008.
Functional Fitness: COPD/Asthma (DVD), Suzanne Andrews, 2009.
100 Questions & Answers About COPD, Campion E. Quinn, Jones & Bartlett Publishers, 2005.
Forward any comments to:
Lawrence Martin, M.D.
Asthma home page |
10 Common Errors in Asthma Management |
Chronic Cough home page |
Lakesidepress home page |
Subject Index for all web
sites
Copyright © Lawrence Martin, M.D.
Initially posted December 2008; Last revised December 21, 2012
Oral prednisone has potential serious side effects when take long term. Taken short term, which I define as less than two weeks, the drug causes few if any side effects. Furthermore, a short burst of prednisone can often obviate hospitalization for a "COPD exacerbation." If hospitalized for this condition the treatment will usually involve a much higher does of steroids than might have prevented the flare up in the first place.
There is no "right dose" of prednisone for a COPD exacerbation. I usually prescribe the
following for adults:
Prednisone 20 mg tablets:
One three times a day for 3 days, followed by
One twice a day for 3 days, followed by
One a day for 5 days, total 20 tablets over 11 days.
8. Missing the diagnosis of COPD because of
"clear lung fields" on exam.
A patient can have clear lungs if the exam is done only during quiet breathing. The examiner places the stethoscope over the lungs and pronounces them 'clear - no wheezing.' In fact wheezing may be heard, but only after a deep breath followed by a forceful exhalation. Here the problem is simply an inexperienced care giver (physician, nurse practioner or other health aide). Many asthmatics and COPD patients with 'clear lung fields' on exam in fact have bad lungs, with wheezes heard only at
the end of a forced exhalation.
9. Over-using antibiotics to treat COPD exacerbations.
Antibiotics are commonly used in treating COPD, but often bacterial
infection is not the problem, or rather using an antibiotic alone won't fix
the problem. The most common triggers of a COPD exacerbation are probably viral
infections, but all physicians use antibiotics in the initial phase. This is
accepted practice, but again, additional medication is usually needed: invariably bronchodilators and steroids.
10. Not considering environmental factors in managing COPD.
Occupationally-related lung disease
is a common problem, and should always
be considered when evaluating an adult with wheezing or shortness of breath that
is not from heart disease. 2nd hand smoke at home or the workplace doesn't typically cause
COPD, but can aggravate the condition. The work environment can cause chronic, low level wheezing and shortness of breath which, over time, can end up as COPD. While treatment is usually the same regardless of precipitating causes (and smoking is by far the most common),
if triggers can be identified the patient MUST avoid them. This may be
difficult if one's livelihood is causing the problem.
Books About COPD -- for a General Audience
(With links to Amazon.com. Books listed in reverse
order of publication)
Live Your Life with COPD - 52 Weeks of Health, Happiness and Hope.
Jane M. Martin, Infinity Publishing, 2011.
The Complete Guide to Understanding and Living with COPD: From a COPDer's Perspective. R.D. Martin, CreateSpace, 2010.
Positive Options for Living with COPD: Self-Help and Treatment for Chronic Obstructive Pulmonary Disease, Teri Allen, Hunter House, 2010.
COPD for Dummies, Kevin Feiner, MD, Meg Scheider, Books for Dummies, 2008.
Functional Fitness: COPD/Asthma (DVD), Suzanne Andrews, 2009.
100 Questions & Answers About COPD, Campion E. Quinn, Jones & Bartlett Publishers, 2005.
Forward any comments to:
Lawrence Martin, M.D.
Asthma home page |
10 Common Errors in Asthma Management |
Chronic Cough home page |
Lakesidepress home page |
Subject Index for all web
sites
Copyright © Lawrence Martin, M.D.
Initially posted December 2008; Last revised December 21, 2012
Prednisone 20 mg tablets:
One three times a day for 3 days, followed by
One twice a day for 3 days, followed by
One a day for 5 days, total 20 tablets over 11 days.
8. Missing the diagnosis of COPD because of
"clear lung fields" on exam.
A patient can have clear lungs if the exam is done only during quiet breathing. The examiner places the stethoscope over the lungs and pronounces them 'clear - no wheezing.' In fact wheezing may be heard, but only after a deep breath followed by a forceful exhalation. Here the problem is simply an inexperienced care giver (physician, nurse practioner or other health aide). Many asthmatics and COPD patients with 'clear lung fields' on exam in fact have bad lungs, with wheezes heard only at
the end of a forced exhalation.
9. Over-using antibiotics to treat COPD exacerbations.
Antibiotics are commonly used in treating COPD, but often bacterial
infection is not the problem, or rather using an antibiotic alone won't fix
the problem. The most common triggers of a COPD exacerbation are probably viral
infections, but all physicians use antibiotics in the initial phase. This is
accepted practice, but again, additional medication is usually needed: invariably bronchodilators and steroids.
10. Not considering environmental factors in managing COPD.
Occupationally-related lung disease
is a common problem, and should always
be considered when evaluating an adult with wheezing or shortness of breath that
is not from heart disease. 2nd hand smoke at home or the workplace doesn't typically cause
COPD, but can aggravate the condition. The work environment can cause chronic, low level wheezing and shortness of breath which, over time, can end up as COPD. While treatment is usually the same regardless of precipitating causes (and smoking is by far the most common),
if triggers can be identified the patient MUST avoid them. This may be
difficult if one's livelihood is causing the problem.
Books About COPD -- for a General Audience
(With links to Amazon.com. Books listed in reverse
order of publication)
Live Your Life with COPD - 52 Weeks of Health, Happiness and Hope.
Jane M. Martin, Infinity Publishing, 2011.
The Complete Guide to Understanding and Living with COPD: From a COPDer's Perspective. R.D. Martin, CreateSpace, 2010.
Positive Options for Living with COPD: Self-Help and Treatment for Chronic Obstructive Pulmonary Disease, Teri Allen, Hunter House, 2010.
COPD for Dummies, Kevin Feiner, MD, Meg Scheider, Books for Dummies, 2008.
Functional Fitness: COPD/Asthma (DVD), Suzanne Andrews, 2009.
100 Questions & Answers About COPD, Campion E. Quinn, Jones & Bartlett Publishers, 2005.
Forward any comments to:
Lawrence Martin, M.D.
Asthma home page |
10 Common Errors in Asthma Management |
Chronic Cough home page |
Lakesidepress home page |
Subject Index for all web
sites
Copyright © Lawrence Martin, M.D.
Initially posted December 2008; Last revised December 21, 2012
9. Over-using antibiotics to treat COPD exacerbations.
Antibiotics are commonly used in treating COPD, but often bacterial
infection is not the problem, or rather using an antibiotic alone won't fix
the problem. The most common triggers of a COPD exacerbation are probably viral
infections, but all physicians use antibiotics in the initial phase. This is
accepted practice, but again, additional medication is usually needed: invariably bronchodilators and steroids.
10. Not considering environmental factors in managing COPD.
Occupationally-related lung disease
is a common problem, and should always
be considered when evaluating an adult with wheezing or shortness of breath that
is not from heart disease. 2nd hand smoke at home or the workplace doesn't typically cause
COPD, but can aggravate the condition. The work environment can cause chronic, low level wheezing and shortness of breath which, over time, can end up as COPD. While treatment is usually the same regardless of precipitating causes (and smoking is by far the most common),
if triggers can be identified the patient MUST avoid them. This may be
difficult if one's livelihood is causing the problem.
Books About COPD -- for a General Audience
(With links to Amazon.com. Books listed in reverse
order of publication)
Live Your Life with COPD - 52 Weeks of Health, Happiness and Hope.
Jane M. Martin, Infinity Publishing, 2011.
The Complete Guide to Understanding and Living with COPD: From a COPDer's Perspective. R.D. Martin, CreateSpace, 2010.
Positive Options for Living with COPD: Self-Help and Treatment for Chronic Obstructive Pulmonary Disease, Teri Allen, Hunter House, 2010.
COPD for Dummies, Kevin Feiner, MD, Meg Scheider, Books for Dummies, 2008.
Functional Fitness: COPD/Asthma (DVD), Suzanne Andrews, 2009.
100 Questions & Answers About COPD, Campion E. Quinn, Jones & Bartlett Publishers, 2005.
Forward any comments to:
Lawrence Martin, M.D.
Asthma home page |
10 Common Errors in Asthma Management |
Chronic Cough home page |
Lakesidepress home page |
Subject Index for all web
sites
Copyright © Lawrence Martin, M.D.
Initially posted December 2008; Last revised December 21, 2012
Books About COPD -- for a General Audience(With links to Amazon.com. Books listed in reverse order of publication)Live Your Life with COPD - 52 Weeks of Health, Happiness and Hope. Jane M. Martin, Infinity Publishing, 2011. The Complete Guide to Understanding and Living with COPD: From a COPDer's Perspective. R.D. Martin, CreateSpace, 2010. Positive Options for Living with COPD: Self-Help and Treatment for Chronic Obstructive Pulmonary Disease, Teri Allen, Hunter House, 2010. COPD for Dummies, Kevin Feiner, MD, Meg Scheider, Books for Dummies, 2008.
Functional Fitness: COPD/Asthma (DVD), Suzanne Andrews, 2009.
100 Questions & Answers About COPD, Campion E. Quinn, Jones & Bartlett Publishers, 2005.
|
Forward any comments to: Lawrence Martin, M.D.
Asthma home page | 10 Common Errors in Asthma Management | Chronic Cough home page | Lakesidepress home page | Subject Index for all web sites
Copyright © Lawrence Martin, M.D.
Initially posted December 2008; Last revised December 21, 2012