Easy Blood Gas Quiz
Lawrence Martin, M.D.
Chairman, Dept. of Medicine, UH-Richmond Medical Center
Richmond Hts., Ohio 44143
Clinical Professor of Medicine
Case Western Reserve University School of Medicine, Cleveland
Lakeside Press Home Page
Pulmonary Physiology web sites
This multiple-choice blood gas quiz is an easier version of a
blood quiz posted in 2009. Both blood gas quizzes are based on information presented in my
Pulmonary Physiology web sites, especially
The Four Most Important Equations in Clinical Practice. These equations are shown below in the box. You will need to understand these simple relationships to properly interpret blood gases. An alternative web site for these four equations is at Clinician's Ultimate Reference.
I recommend you answer the each question or set of questions first, before checking the answers. For questions that require any calculation, it is always best to do it on paper rather than in your head. I cannot over emphasize the importance of becoming familiar with relationships expressed in THE FOUR MOST IMPORTANT EQUATIONS (below). When you are ready to check your answers
please click here.
When finished with one or more questions, click here for the answers.
1) Given the following arterial blood gas values:
pH: 7.56
PaCO2: 31 mm Hg
HCO3: 27 mEq/l
PaO2: 56 mm Hg
The most likely acid-base state in this patient?
a) acute respiratory alkalosis
b) chronic respiratory alkalosis
c) respiratory alkalosis and metabolic alkalosis
Below is an 'acid base map' that shows the expected bands for each of the primary acid base disorders. By plotting the above pH and PCO2 on this map, you can gain a better appreciation of the patient's acid-base state. This map is also included in several other acid-base questions. Each time, I recommend you plot the pH and PaCO2. In the answer section you will find a link that shows this map with an arrow indicating the acid-base state.
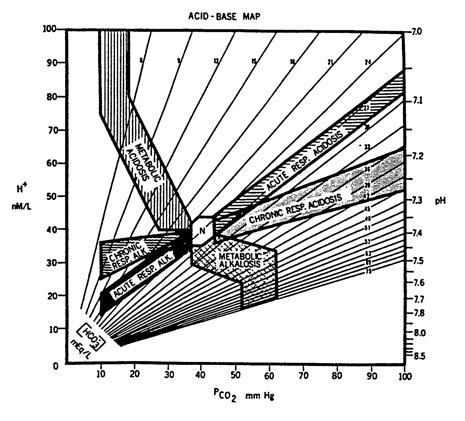
2) A healthy young woman, toward the end of a mile run in the gym, has increased her heart rate by 50% over baseline. At that point, her PaCO2 = 40 mm Hg. This is because:
a) She is not doing any aerobic exercise.
b) She likely has lung disease.
c) Her alveolar ventilation (VA) has increased to match her body's CO2 production (VCO2), so the ratio of VA/VCO2 is normal.
3) Which of the following changes will most increase arterial oxygen delivery?
a) PaO2 from 60 to 95 mm Hg
b) cardiac output from 4 to 5 L/min
c) hemoglobin from 9 to 10 grams%
4) Given the following arterial blood gas values:
pH: 7.40
PaCO2: 20 mm Hg
HCO3: 12 mEq/l
What is the most likely acid-base state in the patient?
a) metabolic acidosis with full compensation
b) respiratory alkalosis with full compensation
c) metabolic acidosis and respiratory alkalosis

5) Which one of the following statements is true about SpO2 as measured by pulse oximeters that utilize two wavelengths of light (i.e., most pulse oximeters in use today)?
a) SpO2 can be normal even when PaCO2 is >200 mm Hg.
b) SpO2 is affected by anemia.
c) SpO2 will differentiate oxyhemoglobin from carboxyhemoglobin.
6) Which of the following statements is true about non-arterial assessment of blood gases?
a) if venous HCO3 is truly abnormal, the patient has some type of acid-base disorder.
b) venous blood from a central line is unreliable for assessing a patient's acid-base state
c) venous blood cannot be used to diagnose carbon monoxide poisoning.
7) Which of the following is a correct statement?
a) If the lungs and heart are normal, then PaO2 is affected only by factors that affect alveolar PO2.
b) Anemia will lower the PaO2.
c) The reason PaO2 falls with increasing altitude is because FIO2 falls.
8) A patient presents with the following arterial blood gases, drawn on room air (FIO2 = .21).
pH: 7.40
PCO2: 40 mm Hg
PO2: 82 mm Hg
HCO3: 24 mEq/L
Which of the following statements is most accurate?
a) The patient does not have an acid-base disorder.b) To determine if there is an acid base disorder you need to know the measured serum bicarbonate, since the HCO3 from blood gases is only a calculation.
c) A patient can have normal blood gases if two metabolic acid-base disorders oppose to give normal bicarbonate.

9) A patient with respiratory failure has the following arterial blood gases (FIO2 = .21, sea level):
pH: 7.20
PCO2: 70 mm Hg
PO2: 60 mm Hg
SaO2: 86%
Which of the following is the most likely cause of these abnormal blood gases?
a) congestive heart failure
b) pneumonia
c) narcotic overdose

10) A 42-year-old man is admitted to the hospital with dehydration and hypotension.
Electrolytes show:
Na+ 165 mEq/L K+ 4.0 mEq/L HCO3 32 mEq/L Cl- 112 mEq/L No arterial blood gas is obtained. Which statement best applies about this patient's acid-base status?
a) Electrolytes indicate the presence of metabolic acidosis.
b) Electrolytes indicate the presence of metabolic alkalosis.
c) Electrolytes indicate the presence of both metabolic acidosis and metabolic alkalosis.
11) A 30-year-old man, previously healthy, is brought to the ED after suffering smoke inhalation. Measured carboxyhemoglobin is 20% and hemoglobin content = 15 gm%. He has following blood gas values:
PaO2: 80 mm Hg (on room air at sea level)
PaCO2: 32 mm Hg
pH: 7.34
SaO2: 96% (calculated)
Exam shows clear lungs to auscultation and his chest x-ray is normal. From this information alone, you can determine that:
a) His actual SaO2 is much lower than the calculated value.
b) There is no lung abnormality present, though pulmonary disease could develop in the ensuing 24 hours.
c) He has a mild metabolic acidosis associated with an increased anion gap.

12) Below are two sets of blood gases:
Patient A: pH 7.48, PaCO2 34 mm Hg, PaO2 85 mm Hg, SaO2 95%, Hemoglobin 7 gm%
Patient B: pH 7.32, PaCO2 74 mm Hg, PaO2 55 mm Hg, SaO2 85%, Hemoglobin 15 gm%
Which is the most correct statement?
a) B is more hypoxemic because PaO2 is lower than A.
b) B is more hypoxemic because SaO2 is lower than A.
c) A is more hypoxemic because O2 content is lower than B.
13) State which one of the following situations would be expected to lower a patient's arterial PO2.
a) anemia
b) carbon monoxide poisoning
c) lung disease with increased ventilation-perfusion imbalance
14) A 40 year-old patient is admitted to the ICU with the following lab values:
pH 7.40
Na: 149 mEq/L
Which statement best describes these values?
a) Normal electrolytes, normal blood gases
b) Metabolic acidosis
c) Metabolic acidosis and metabolic alkalosis
15) Which one of the following statements about cyanosis is correct?
a) For cyanosis to manifest there needs to be 5 gm% of deoxygenated hemoglobin
in the arterial blood. b) Patients with anemia manifest cyanosis at higher SaO2 values than patients without anemia. c) Cyanosis can be caused by excess methemoglobin, which is HbFe+3.
16) Since the early 1980s, climbers have summited Mt. Everest without supplemental oxygen. Since the barometric pressure on the summit is only 253 mm Hg, summiting (without extra O2) has only been possible due to prolonged acclimitization at altitude and profound hyperventilation.
Indeed, if a a climber maintained PaCO2 of 40 mm Hg and an alveolar-arterial
PO2 difference of 5 mm Hg, what would be his/her theoretical PaO2?
a) 25 mm Hg b) 5 mm Hg c) -10 mm Hg 17) Which of the following statements is true about excess carbon monoxide?
a) shifts the oxygen dissociation curve to the right
b) lowers the PaO2
c) lowers the oxygen saturation
18) A mountain climber ascends from sea level to 18,000 feet over a two day period, without
supplemental oxygen. With ascent which one of the following should alaways decrease? a) FIO2 b) Barometric pressure c) pH 19) A 35 year old male patient is being treated in a hyperbaric chamber for carbon monoxide poisoning. Assume he is receiving 100% oxygen (FIO2 = 1.0) at TWO atmospheres of pressure and he has no lung disease. His PaO2 is approximately: a) 200 mm Hg b) 600 mm Hg c) 1400 mm Hg 20) While the following conditions could possibly be managed without measuring arterial blood gases, in which one would blood gases be most helpful?
a) A 17-year-old-high school student who presents to the ED with hyperpnea and tachypnea; history reveals he became "excited" during a church service. He has some tetanic contractions of his hands, his lungs are clear and pulse oxygen saturation is 98% on room air.
b) A 68-year-old hypertensive patient has been feeling "weak" for a few days. She has been taking her anti-hypertensive medications. Electrolyte measurements show: 21) Which of the following is not a component of the PaCO2 equation?
a) Metabolic CO2 production
b) Alveolar ventilation
c) Oxygen uptake
22) The limit of human hyperventilation is a PaCO2 of about 8 mm Hg.
What is the highest PaO2 (mm Hg) a patient with normal lungs
could achieve breathing room air (FIO2=.21) at sea level?
a) 100
b) 122
c) 135
23) A 45 year-old-man is treated in a hyperbaric chamber for severe
carbon monoxide toxicity. Assume he is breathing 100% oxygen at 3 atmospheres
of pressure, that he has normal lungs, and that hemoglobin=15 gm%,
carboxyhemoglobin=40%. What is his approximate arterial oxygen content in ml/dl? a) 10
b) 13
c) 17
24) Which one of the following statements is true? a) If nothing else changes, as PaCO2 goes up alveolar PO2 and arterial PO2 go down. b) PaO2 is inversely related to blood pH: as pH goes up PaO2 also increases. c) If PaCO2 increases while HCO3- remains unchanged, pH also goes up. 25) Which one of the following sets of blood gas values most likely
represents a lab or transcription error? (PaCO2 and PaO2 in mm Hg, HCO3 in mEq/L,
SaO2 in %. Assume all blood gases drawn at sea level.)
BLOOD GASES
PCO2: 38 mm Hg
HCO3: 24 mEq/L
PO2: 88 mm Hg (on room air)
ELECTROLYTES, BUN & CREATININE
K: 3.8 mEq/L
Cl: 100 mEq/L
HCO3: 24 mEq/L
BUN: 110 mg%
Creatinine: 8.7 mg%

Na+ 148 mEq/L K+ 4.0 mEq/L HCO3 24 mEq/L Cl- 102 mEq/L
c) A 24-year-old insulin-dependent diabetic comes to the ED, complaining of lethargy; she has not used insulin in several days. Her pulse oximeter oxygen saturation on room air is 98%. Lab values show:
Glucose 750 mg% Na+ 135 mEq/L K+ 4.5 mEq/L HCO3 10 mEq/L Cl- 100 mEq/L Urine 4+ ketones
pH PaCO2 HCO3 PaO2
SaO2 FIO2
a) 7.40 75 45 70 75 0.21
b) 7.22 20 8 160 98 0.50
c) 7.59 25 23 60 90 0.28