Easy Blood Gas Quiz: Questions with Answers
Lawrence Martin, M.D.
Chairman, Dept. of Medicine, UH-Richmond Medical Center
Richmond Hts., Ohio 44143
Clinical Professor of Medicine
Case Western Reserve University School of Medicine, Cleveland
Lakeside Press Home Page
Pulmonary Physiology web sites
This web site contains all the questions of the
Easy Blood Gas Quiz
with answers. Also shown below is the box containing
The Four Most Important Equations in Clinical Practice.
BLOOD GAS QUESTIONS WITH ANSWERS
1) Given the following arterial blood gas values:
pH: 7.56
PaCO2: 31 mm Hg
HCO3: 27 mEq/l
PaO2: 56 mm Hg
The most likely acid-base state in this patient?
a) acute respiratory alkalosis
b) chronic respiratory alkalosis
c) respiratory alkalosis and metabolic alkalosis
Answer: c The subject is hyperventilating with an elevated pH, indicating respriratory alkalosis. However, with hyperventilation bicarbonate goes down from the simple excretion of CO2 in the hydration equation; CO2 + H2O <----> HCO3- + H+. As CO2 is excreted both bicarbonate and hydrogen ion fall. With simple hyperventilation and no other acid base disorder the HCO3- should fall slightly from the normal value of 24 mEq/L, and be about 22-23 mEq/L. In this example it is 27 mEq/L, indicating a comcomitant mild metabolic alkalosis.
Below is an 'acid base map' that shows the expected bands for each of the primary acid base disorders. By plotting the above pH and PCO2 on this map, you can see that the blood gas falls between the bands for respiratory alkalosis and metabolic alkalosis. This map is also included in answers to other acid-base questions. Each time, I recommend you plot the pH and PaCO2, then click on the link below the map; it will take you to a pdf file that shows the same map with an arrow pointing to the patient's acid-base state.
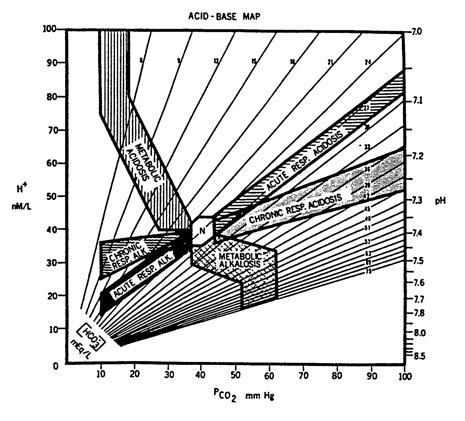
Click here for plot of acid-base state.
2) A healthy young woman, toward the end of a mile run in the gym, has increased her heart rate by 50% over baseline. At that point, her PaCO2 = 40 mm Hg. This is because:
a) She is not doing any aerobic exercise.
b) She likely has lung disease.
c) Her alveolar ventilation (VA) has increased to match her body's CO2 production (VCO2), so the ratio of VA/VCO2 is normal.
Answer: c Hyperventilation is defined excess alveolar ventilation for CO2 production, which always leads to a low PaCO2 (if the subject starts from normal value of @40 mm Hg). It is NOT the same as tachypnea or hyperpnea. Since this healthy young woman has increased her heart rate with exercise, she has surely increased her CO2 production as well, and as a result her respiratory rate to blow it off. Thus she is tachypneic and or hyperpneic in order to increase her alveolar ventilation, which is necessary to excrete her excess metabolic CO2 production. She should NOT be hyperventilating though, as her increase in CO2 production will be balanced by increased alveolar ventilation, leaving PaCO2 unchanged. For further discussion see The Four Most Important Equations in Clinical Practice.
3) Which of the following changes will most increase arterial oxygen delivery?
a) PaO2 from 60 to 95 mm Hg
b) cardiac output from 4 to 5 L/min
c) hemoglobin from 9 to 10 grams%
Answer: b Oxygen delivery is oxygen content x cardiac output.
a) This change in PaO2 will increase SaO2 from about 90% to 98%, in effect increasing CaO2 about 9% and O2 delivery about the same.
b) This is a 25% increase in cardiac output, which will increase oxygen delivery 25%.
c) This is an 11% increase in oxygen content, which will increase O2 delivery the same percentage, 11%.
4) Given the following arterial blood gas values:
pH: 7.40
PaCO2: 20 mm Hg
HCO3: 12 mEq/l
What is the most likely acid-base state in the patient?
a) metabolic acidosis with full compensation
b) respiratory alkalosis with full compensation
c) metabolic acidosis and respiratory alkalosis
Answer: c A normal pH with abnormal PaCO2 or HCO3 indicates two or more acid base disorders. Full compensation (back to baseline) is never achieved with a single acid-base disorder.

Click here for plot of acid-base state.
5) Which one of the following statements is true about SpO2 as measured by pulse oximeters that utilize two wavelengths of light (i.e., most pulse oximeters in use today)?
a) SpO2 can be normal even when PaCO2 is >200 mm Hg.
b) SpO2 is affected by anemia.
c) SpO2 will differentiate oxyhemoglobin from carboxyhemoglobin.
Answer: a This is true IF the patient is receiving supplemental O2, which washes out the nitrogen and increases the PaO2, but doesn't affect PaCO2. Thus a patient can have a very high PaCO2 and a normal or even increased PaO2. If all that is being monitored in this situation is pulse oximetry, you can miss a severe respiratory acidosis.
6) Which of the following statements is true about non-arterial assessment of blood gases?
a) if venous HCO3 is truly abnormal, the patient has some type of acid-base disorder.
b) venous blood from a central line is unreliable for assessing a patient's acid-base state
c) venous blood cannot be used to diagnose carbon monoxide poisoning.
Answer: a If any of the 3 components of the Henderson-Hasselbalch equation are abnormal, the patient has an acid-base disorder. Venous blood from a central line IS reliable for assesing a patient's acid-base status in any stable patient; the differences between venous and arterial pH and PaCO2 are small. COHb is the same in both venous and arterial blood, so either source can be used to diagnose CO poisoning.
7) Which of the following is a correct statement?
a) If the lungs and heart are normal, then PaO2 is affected only by factors that affect alveolar PO2.
b) Anemia will lower the PaO2.
c) The reason PaO2 falls with increasing altitude is because FIO2 falls.
Answer: a. Anemia does not lower the PaO2, only the hemoglobin content. PaO2 falls with altitude because of the drop in barometric pressure. The FIO2 is a constant 0.21 at all breathable altitudes.
8) A patient presents with the following arterial blood gases, drawn on room air (FIO2 = .21).
pH: 7.40
PCO2: 40 mm Hg
PO2: 82 mm Hg
HCO3: 24 mEq/L
Which of the following statements is most accurate?
a) The patient does not have an acid-base disorder.b) To determine if there is an acid base disorder you need to know the measured serum bicarbonate, since the HCO3 from blood gases is only a calculation.
c) A patient can have normal blood gases if two metabolic acid-base disorders oppose to give normal bicarbonate.
Answer: c Metabolic acidosis and metabolic alkalosis can co-exist, so the patient ends up with a normal HCO3 and PaCO2. Serum electrolytes can make the diagnosis by showing increased anion gap; in the presence of normal HCO3, increased AG would indicate the two disorders. See also Question 14.

Click here for plot of acid-base state.
9) A patient with respiratory failure has the following arterial blood gases (FIO2 = .21, sea level):
pH: 7.20
PCO2: 70 mm Hg
PO2: 60 mm Hg
SaO2: 86%
Which of the following is the most likely cause of these abnormal blood gases?
a) congestive heart failure
b) pneumonia
c) narcotic overdose
Answer: c All of these conditions can lead to respiratory acidosis, but the first two conditions would be expected to give an increased A-a O2 difference ('A-a gradient'). Alveolar PO2 in this example is .21(760-43) - 1.2(PaCO2) = 150-84 = 66 mm Hg. Thus the (PAO2 - PaO2), or so-called 'A-a gradient' is normal at about 6 mm Hg. Since the only diagnosis not directly involving the lung parenchyma is narcotic dose, that is the best answer.

Click here for plot of acid-base state.
10) A 42-year-old man is admitted to the hospital with dehydration and hypotension.
Electrolytes show:
Na+ 165 mEq/L K+ 4.0 mEq/L HCO3 32 mEq/L Cl- 112 mEq/L No arterial blood gas is obtained. Which statement best applies about this patient's acid-base status?
a) Electrolytes indicate the presence of metabolic acidosis.
b) Electrolytes indicate the presence of metabolic alkalosis.
c) Electrolytes indicate the presence of both metabolic acidosis and metabolic alkalosis.
Answer: c His anion gap is Na - (HCO3 + Cl) = 165 - (32+112) = 21, indicating a metabolic acidosis. His bicarbonate gap is (Na - Cl - 39) = 165 - 112 - 39 = 14, which indicates a metabolic alkalosis. Note that it is not necessary to actually calculate a bicarbonate gap, since the elevated serum HCO3 (32 mEq/L) in the presence of anion gap acidosis is enought to signify concomitant metabolic alkalosis. Of course without blood gas measurements you don't know which process is predominant, but it is clear from the electrolytes that both metabolic disorders are present.
11) A 30-year-old man, previously healthy, is brought to the ED after suffering smoke inhalation. Measured carboxyhemoglobin is 20% and hemoglobin content = 15 gm%. He has following blood gas values:
PaO2: 80 mm Hg (on room air at sea level)
PaCO2: 32 mm Hg
pH: 7.34
SaO2: 96% (calculated)
Exam shows clear lungs to auscultation and his chest x-ray is normal. From this information alone, you can determine that:
a) His actual SaO2 is much lower than the calculated value.
b) There is no lung abnormality present, though pulmonary disease could develop in the ensuing 24 hours.
c) He has a mild metabolic acidosis associated with an increased anion gap.
Answer: a Given 20% carboxyhemoglobin, his actual SaO2 (which is the % of hemoglobin bound to oxygen) cannot be more than 80%. As to: b), a lung abnormality could be present, especially since his (PAO2-PaO2) is somewhat increased; c) no electrolyte information is given, so you don't know if he has an increased anion gap.

Click here for plot of acid-base state.
12) Below are two sets of blood gases:
Patient A: pH 7.48, PaCO2 34 mm Hg, PaO2 85 mm Hg, SaO2 95%, Hemoglobin 7 gm%
Patient B: pH 7.32, PaCO2 74 mm Hg, PaO2 55 mm Hg, SaO2 85%, Hemoglobin 15 gm%
Which is the most correct statement?
a) B is more hypoxemic because PaO2 is lower than A.
b) B is more hypoxemic because SaO2 is lower than A.
c) A is more hypoxemic because O2 content is lower than B.
|
Answer: c
Hypoxemia means low oxygen content in the blood. If you don't know the oxygen content, you might use PaO2 and/or SaO2 as a surrogate for assessing hypoxemia, but here you do have oxygen content information. The oxygen contents are (excluding contribution of dissolved O2): Patient A: 1.34 x 7 x .95 = 8.91 ml O2/dl Patient B: 1.34 x 15 x .85 = 17.09 ml O2/dl Clearly, Patient A is more hypoxemic despite having a higher PaO2 and SaO2 than Patient B. |
13) State which one of the following situations would be expected to lower a patient's arterial PO2.
a) anemia
b) carbon monoxide poisoning
c) lung disease with increased ventilation-perfusion imbalance
Answer: c PaO2 is unaffected by anemia or anything to do with hemoglobin binding, including COHb. PaO2 is a function of what's inhaled (Alveolar PO2) and the state of lung architecture; the latter is defined by ventilation-perfusion abnormality (which includes right to left shunting). The most common cause of low PaO2 is ventilation-perfusion imbalance -- the physiologic cause of hypoxemia in virtually all acute and chronic lung diseases.
14) A 40 year-old patient is admitted to the ICU with the following lab values:
pH 7.40
Na: 149 mEq/L
Which statement best describes these values?
a) Normal electrolytes, normal blood gases
b) Metabolic acidosis
c) Metabolic acidosis and metabolic alkalosis
Electrolytes are not normal, since the anion gap is increased:
AG = 149 - (100+24) = 25. Thus, there is at least a metabolic acidosis.
Furthermore, since HCO3 is "normal" at 24, despite an increased anion gap, there
must also be a metabolic alkalosis. See also Question 8.
15) Which one of the following statements about cyanosis is correct?
a) For cyanosis to manifest there needs to be 5 gm% of deoxygenated hemoglobin
in the arterial blood. b) Patients with anemia manifest cyanosis at higher SaO2 values than patients without anemia. c) Cyanosis can be caused by excess methemoglobin, which is HbFe+3.
Methemoglobin can cause cyanosis with a normal PaO2. For cyanosis to manifest there
needs to be 5 gm% deoxygenated hemoglobin in the capillaries. Patients without
anemia show cyanosis sooner than anemic patients, because they have more hemoglobin that can become doxygenated. See e-medicine topic
on cyanosis
16) Since the early 1980s, climbers have summited Mt. Everest without supplemental oxygen. Since the barometric pressure on the summit is only 253 mm Hg, summiting (without extra O2) has only been possible due to prolonged acclimitization at altitude and profound hyperventilation.
Indeed, if a a climber maintained PaCO2 of 40 mm Hg and an alveolar-arterial
PO2 difference of 5 mm Hg, what would be his/her theoretical PaO2?
a) 25 mm Hg b) 5 mm Hg c) -10 mm Hg
Here you use the alveolar gas equation:
PAO2 = .21 (253-47) - 1.2 (40) = 43 - 48 = - 5 mm Hg
Since the alveolar-arterial PO2 difference is 5 mm Hg, that
would make the arterial PO2 (PaO2) = -10 mm Hg!
Climbers have summited without supplemental O2 by virtue of profound, sustained
hyperventilation, to level of @ 7 mm Hg. Thus
PAO2 = .21 (253-47) - 1.2 (7) = 43 - 8 = 35 mm Hg
Again, since the alveolar-arterial PO2 difference is 5 mm Hg, that
would gives a PaO2 on the summit of @ 30 mm Hg - extremely low but survivable.
17) Which of the following statements is true about excess carbon monoxide?
a) shifts the oxygen dissociation curve to the right
b) lowers the PaO2
c) lowers the oxygen saturation
CO shifts the O2 dissociation curve leftward, lowers SaO2 (and therefore oxygen content) and does not affect PaO2. PaO2 is affected only the the PAO2 and lung architecture.
18) A mountain climber ascends from sea level to 18,000 feet over a two day period, without
supplemental oxygen. With ascent which one of the following should always decrease? a) FIO2 b) Barometric pressure c) pH
As discussed in a previous question, FIO2 is the same at all breathable altitudes.
The barometric pressure always falls with altitude, and as a consequence
PAO2 and PaO2 fall and the climber hyperventilates. As result of hyperventilation the pH increases (respiratory alkalosis).
19) A 35 year old male patient is being treated in a hyperbaric chamber for carbon monoxide poisoning. Assume he is receiving 100% oxygen (FIO2 = 1.0) at TWO atmospheres of pressure and he has no lung disease. His PaO2 is approximately: a) 200 mm Hg b) 600 mm Hg c) 1400 mm Hg
Using the alveolar gas equation, with FIO2 = 1.0 and barometric pressure is twice that of sea level (1620 mm Hg), we obtain: 20) While the following conditions could possibly be managed without measuring arterial blood gases, in which one would blood gases be most helpful?
a) A 17-year-old-high school student who presents to the ED with hyperpnea and tachypnea; history reveals he became "excited" during a church service. He has some tetanic contractions of his hands, his lungs are clear and pulse oxygen saturation is 98% on room air.
b) A 68-year-old hypertensive patient has been feeling "weak" for a few days. She has been taking her anti-hypertensive medications. Electrolyte measurements show:
The patient has an anion gap metabolic acidosis and a metabolic alkalosis (see
also Questions 8 and 14). Blood gases will be helpful to determine which disorder
is predominant. Patients a and c can be treated without measuring blood gases.
Patient c, in particular, reflects a typical case of diabetic ketoacidosis, with
anion gap of 25 mEq/L. It is not necessary to measure or follow blood gases in
such a patient, as long as there is response to insulin along with a rise in AG and
fall in serum HCO3.
21) Which of the following is not a component of the PaCO2 equation?
a) Metabolic CO2 production
b) Alveolar ventilation
c) Oxygen uptake
The PaCO2 equation, PaCO2 = VCO2/VA, does not include anything about oxygen uptake.
22) The limit of human hyperventilation is a PaCO2 of about 8 mm Hg.
What is the highest PaO2 (mm Hg) a patient with normal lungs
could achieve breathing room air (FIO2=.21) at sea level?
a) 100
b) 122
c) 135
The abbreviated alveolar gas equation gas can be used to answer this question:
PAO2 = FIO2 (BP - 47) - 1.2 (PaCO2)
PAO2 = .21 (760-47) - 1.2 (8)
PAO2 = 149.7 - 9.6 = 140.1. Thus the alveolar PO2 is 140, but arterial
PO2 will be somewhat lower due to the normal PAO2 - PaO2 difference. Assuming a
normal PAO2-PaO2 of 5 mm Hg, the highest value would be about 135 mm Hg.
23) A 45 year-old-man is treated in a hyperbaric chamber for severe
carbon monoxide toxicity. Assume he is breathing 100% oxygen at 3 atmospheres
of pressure, that he has normal lungs, and that hemoglobin=15 gm%,
carboxyhemoglobin=40%. What is his approximate arterial oxygen content in ml/dl? a) 10
b) 13
c) 17
Here you use the oxygen content equation. In this situation you need
to include the contribution by PaO2, since it is considerably higher in a hyperbaric
environment. Since the subject has normal lungs, PaO2 should be
3x normal PaO2 while breathing 100% oxygen. PaO2 on 100% oxygen with normal lungs should
be around 600 mm Hg. Therefore at 3 atmospheres, PaO2 should be around 1800 mm Hg.
Also, with 3 atmospheres of pressure at 100% oxygen,
all the available hemoglobin binding sites will be fully saturated.
However, since 40% of the sites are bound with CO (COHb = 40%),
the actual SaO2 will only be 60%. Thus:
CaO2 = 1.34 x Hgb x SaO2 + (.003 x PaO2)
CaO2 = 1.34 x 15 x .60 + (.003 x 1800)
CaO2 = 12.06 + 5.4 = 17.46 ml O2/dl
24) Which one of the following statements is true? a) If nothing else changes, as PaCO2 goes up alveolar PO2 and arterial PO2 go down. b) PaO2 is inversely related to blood pH: as pH goes up PaO2 also increases. c) If PaCO2 increases while HCO3- remains unchanged, pH also goes up.
As PaCO2 goes up alveolar PO2 (PAO2) goes down, as shown by the alveolar gas equation. As a
consequence, PaO2 also goes down. The other two relationships are incorrect. There is no equation relating PaO2 to pH. In the changes described in c), pH would go down (the patient would become acidotic).
25) Which one of the following sets of blood gas values most likely
represents a lab or transcription error? (PaCO2 and PaO2 in mm Hg, HCO3 in mEq/L,
SaO2 in %. Assume all blood gases drawn at sea level.)
A lab or transcription error is evident when the values do not fit
known physiology. a) doesn't fit because the PaO2 is too high for this
degree of hypoventilation:
Alveolar PO2 = .21 (760 - 47) - 1.2 (75) = 150-90 = 60 mm Hg. In a clinical situation
you would never have a PaO2 of 70 mm Hg when alveolar PO2 is only 60 mm Hg. (This could
theoretically happen in a sudden decompression at altitude, in which case oxygen
would be leaving the blood to enter the atmosphere. In that situation
you wouldn't be checking blood gases!)
b) reflects severe metabolic acidosis with increased PaO2 on a high FIO2.
BLOOD GASES
PCO2: 38 mm Hg
HCO3: 24 mEq/L
PO2: 88 mm Hg (on room air)
ELECTROLYTES, BUN & CREATININE
K: 3.8 mEq/L
Cl: 100 mEq/L
HCO3: 24 mEq/L
BUN: 110 mg%
Creatinine: 8.7 mg%
Answer: c

Click here for plot of acid-base state.
Answer: c
Answer: c
Answer: c
Answer: b
Answer: c
PAO2 = 1.0 (1520 - 47) - PaCO2.
(Note that with 100% O2 the factor 1.2 is not operative). Assuming a normal PaCO2 of 40 mm Hg, this will give 1433 mm Hg. Accounting for some normal increase in A-a gradient that occurs when breathing 100% O2, his PaO2 will be about 1400 mm Hg.
Na+ 148 mEq/L K+ 4.0 mEq/L HCO3 24 mEq/L Cl- 102 mEq/L
c) A 24-year-old insulin-dependent diabetic comes to the ED, complaining of lethargy; she has not used insulin in several days. Her pulse oximeter oxygen saturation on room air is 98%. Lab values show:
Glucose 750 mg% Na+ 135 mEq/L K+ 4.5 mEq/L HCO3 10 mEq/L Cl- 100 mEq/L Urine 4+ ketones
Answer: b
Answer: c
Answer: c
Answer: c
Answer: a
pH PaCO2 HCO3 PaO2
SaO2 FIO2
a) 7.40 75 45 70 75 0.21
b) 7.22 20 8 160 98 0.50
c) 7.59 25 23 60 90 0.28
Answer: a
c) reflects hyperventilation with some hypoxemia.